St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
“Why are you studying influenza at St. Jude?” is a question that 91-year-old Robert Webster, PhD, Department of Infectious Diseases emeritus member, was asked repeatedly throughout his career.
Webster created and grew a globe-spanning influenza research program at St. Jude. The researchers he trained and inspired continue his legacy, staying one step ahead of pathogens for the children of St. Jude — and the world at large.
As for the skepticism about why he chose to do his work at St. Jude, Webster has a clear logic to support that decision. “Immunosuppression, leading to infections such as influenza, mumps, measles, and the common cold, was the real threat to our kids at the time,” he said.
When Webster joined the St. Jude faculty in 1969, cure rates for pediatric cancers were improving drastically. One supportive care factor that improved those successful treatments even further was a better understanding of how to counter infectious disease threats to patients.
When it came to understanding the flu, one of the first steps was figuring out where new flu strains in humans came from. Over 50 years ago, no one knew where the annual seasonal flu viruses originated. Several animal reservoirs were being considered then, and Webster published an article describing the potential relationship between bird flu and human flu in Nature in 1967. Webster and his colleague Graeme Laver, PhD, noticed a pattern of birds in Australia succumbing to mysterious illnesses, prompting them to test seabirds at the Great Barrier Reef. Initial findings were positive for influenza virus and published in the Bulletin of the World Health Organization in 1972, along with another article describing how human pandemic flu may have arisen from an avian virus.
Zeroing in on wild birds by expanding specimen collections to the United States and Canada was a stroke of collaborative brilliance that prompted Webster’s biggest breakthrough in identifying the reservoir of seasonal flu viruses. A visiting Russian scientist found that the influenza virus replicates in ducks’ intestines.

The pandemic H5N1 is better adapted to wild birds than any other highly pathogenic flu viruses we’ve ever seen . . . This is more evidence that these viruses are continually evolving. The real drivers of that evolution occur at the interface of wild and domestic birds, then domestic birds move into urban areas, such as live poultry markets, and that’s where they interface with humans.
Host-Microbe Interactions, co-director of the Center of Excellence for Influenza Research and Response
“It was one of those ‘eureka’ moments,” Webster explained. “The virus was not in the respiratory tract — we’d been looking in the wrong end of the bird for years.” The landmark result was published in 1976 in the Journal of General Virology with a follow-up article in Virology.
Partially due to these findings, in 1975, St. Jude was designated the third (of now seven) World Health Organization (WHO) Collaborating Center. With that came the WHO Global Influenza Surveillance and Response System (GISRS), enabling researchers to actively contribute to global surveillance and control of the virus.
Following the flu in fowl
“Dr. Webster’s projects are probably the longest-running animal flu surveillance programs in the world,” said Richard Webby, PhD, Department of Host-Microbe Interactions, who now oversees several of these programs himself. “We have partners worldwide that we work with to collect samples in decades-long collaborations he established.”
Webby, a former postdoctoral fellow in the Webster lab, follows in his mentor’s footsteps as a principal investigator at St. Jude, studying avian and human flu and expanding the globe-spanning research program and its collaborations. Recently, one of these collaborations identified the ancestors of the current H5N1 influenza strain killing birds in the Americas — and why it is spreading so fast.
“The pandemic H5N1 is better adapted to wild birds than any other highly pathogenic flu viruses we’ve ever seen,” explained Webby, corresponding author of a Nature Communications article reporting the findings from this study.
The transmissibility appears to be helped by access to a different assortment of virus genes in North American wild birds. This has produced a more transmissible and virulent strain that can enter the brains of infected animals.
-
Slide activated
A St. Jude scientist pipettes in the lab.
-
Slide activated
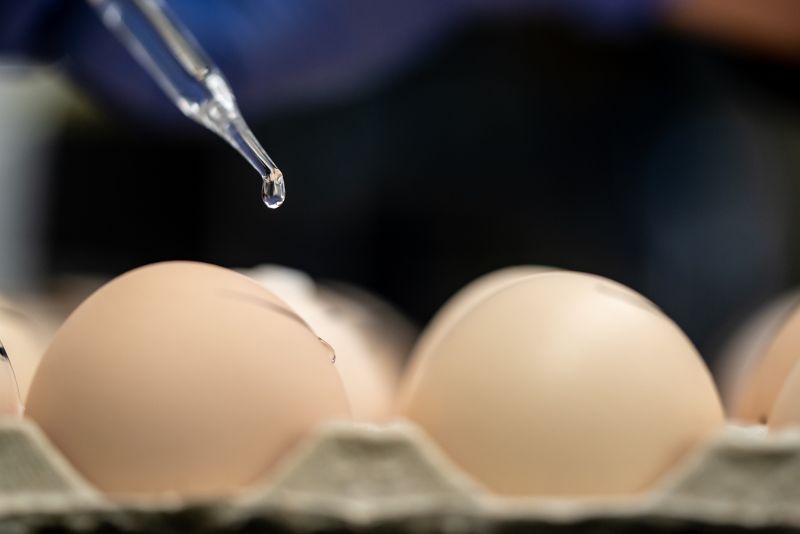
A researcher examines eggs before influenza innoculation.
-
Slide activated
Karlie Woodard, Host Microbe Interactions, inoculates an egg with influenza.
-
Slide activated
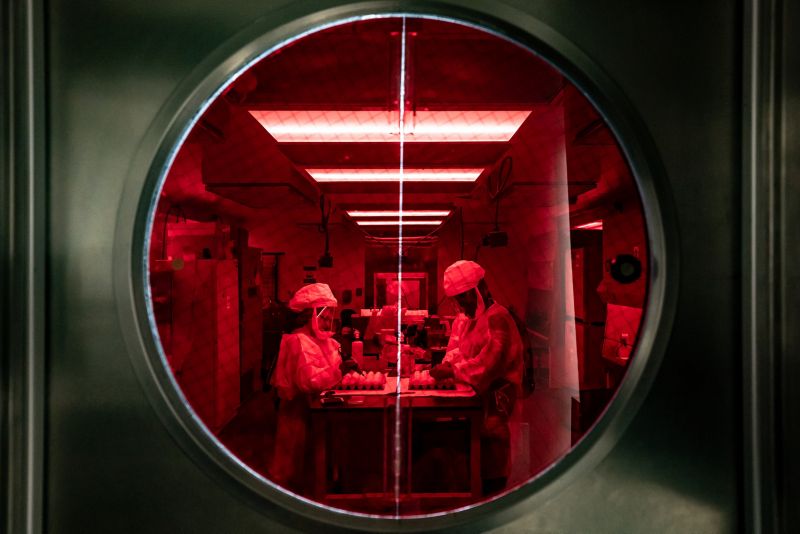
Jeri Carol Crumpton and Jasmine Turner, Host-Microbe Interactions, prepare eggs for influenza inoculation in a Biosafety Level 2+ lab.
-
Slide activated
Crumpton and Turner prepare eggs for influenza inoculation in a Biosafety Level 2+ lab behind a high-security door with a red circadian-rhythm preserving window.
Another collaboration, led by Webby and published in Emerging Microbes and Infection, showed the connection between wild birds, domesticated free-range ducks, and live poultry markets in Bangladesh and the emergence of a novel avian influenza virus.
“This is more evidence that these viruses are continually evolving,” Webby said. “The real drivers of that evolution occur at the interface of wild and domestic birds, then domestic birds move into urban areas, such as live poultry markets, and that’s where they interface with humans.”
Jumping from birds to mammals
Before proving bird flu could jump into humans, Webster first showed avian and mammalian influenza viruses could mix. Influenza virus genomes comprise eight independent segments. When multiple influenza viruses with different segments infect the same cell, they can hybridize by mixing those segments in their progeny. Webster showed that these hybrids can occur naturally in birds and swine in a study published in Virology in 1971.
The true proof of avian-to-human transmission was uncovered when a child succumbed to an influenza virus in May 1997 in Hong Kong. Seventeen more cases followed.
“When Dr. Webster and his collaborators went to Hong Kong and identified the virus,” explained Webby, “it was the first time anyone realized people could be directly infected with these bird flu viruses. It changed the world of influenza virology.” It was a result that was fittingly published in The Lancet later that year. Webster received a National Institutes of Health (NIH) grant to uncover how these viruses were maintained and evolved.
In November and December 1997, Hong Kong experienced another outbreak of avian-to-human flu. Webster was quickly able to find infected birds in the live poultry market again. The findings were published in Nature, further cementing St. Jude as a world leader in influenza research.

Having the Center for Excellence gives us the resources to do those high-risk, high-reward experiments that, if they work, will move the field forward and change textbooks. We can try novel, untested approaches and ideas, and if these projects work, we can move them forward to develop new basic understanding, technology, assays, or therapies.
Host-Microbe Interactions, co-director of the Center of Excellence for Influenza Research and Response
Stacey Schultz-Cherry, PhD, Department of Host-Microbe Interactions, another expert in influenza and other pathogens, explained how Webster’s work in Hong Kong set the stage for the future. “For many years, people always thought that a bird flu strain had to get into a pig and mix with another mammalian virus for it to gain the ability to infect people,” Schultz-Cherry said. “That appears to be true in most cases, but Dr. Webster’s later work showed humans can get these viruses from birds directly.”
Getting ahead of influenza
“That first NIH grant was so tremendously successful that when seven years were up, NIH created the Centers of Excellence for Influenza Research and Response (CEIRR), funding six centers, including St. Jude,” Webster said. “It grew out of St. Jude — we were responsible for these programs starting, and they’re still ongoing today.”
In 2021, the NIH again designated St. Jude a CEIRR through the third iteration of the original CEIRR program. The focus was on developing tools to control epidemic or pandemic influenza while finding ways to minimize the threat of an influenza pandemic.
“Having the Center for Excellence gives us the resources to do those high-risk, high-reward experiments that, if they work, will move the field forward and change textbooks,” said Schultz-Cherry, who co-directs CEIRR with Webby. “We can try novel, untested approaches and ideas, and if these projects work, we can move them forward to develop new basic understanding, technology, assays, or therapies.”
Schultz-Cherry’s work focuses on how the metabolic health of a host explains and may be able to minimize poor infection outcomes.
“Having a high body mass index (BMI) can impact anything from influenza disease severity to susceptibility to infection,” Schultz-Cherry said. Previous research from her lab characterized the difference in response between healthy and obese mice. In a paper published in mBio in 2023, her lab also showed that modern antivirals are less effective in obese mouse models than in fit mice.
Studying the host to understand how a virus will behave can be highly informative for scientists. Webby teamed up with a large collaborative group, including Paul Thomas, PhD, Department of Host-Microbe Interactions, an expert on how the immune system responds to pathogens. Together, they recently published results in Nature Immunology showing that having specific types of innate and adaptive immune cells present up to six months before infection correlates with increased protection from flu symptoms.
-
Slide activated
-
Slide activated
-
Slide activated
-
Slide activated
The researchers found that having a more functionally diverse set of immune cells was correlated with increased protection from flu symptoms. The group identified these cells by comparing the immune cells in the blood of patients who had symptoms of flu infection to those who were asymptomatic or uninfected. Those without symptoms not only had a more functionally diverse set of immune cells, but those cells were also associated with an influenza-specific long-term response, sometimes called the memory response. Patients with symptoms tended to have a more similar set of inflammatory immune cells, which are more likely to be involved in a nonspecific, functionally narrow and short-term response. The work helps answer the age-old question of why some people get sick with infections and others do not.
“By understanding which immune cells are best for fighting the flu, we can start designing vaccines to push for those populations that are most protective,” said Thomas.

Infants die from many infections that adults don’t die from. The best thing we can do for this is vaccinate. We know this strategy works, but we still don’t understand how the vaccines work very well. We have begun to uncover these mechanisms and can think about how to design more effective vaccines for infants.
Chair, Department of Infectious Diseases
The most powerful protection for influenza remains vaccination, especially in vulnerable groups. The world-leading expert in respiratory syncytial virus and the early immune system, Octavio Ramilo, MD, Department of Infectious Diseases chair, is taking a systems-level approach to explore immune response over time in infants, a group considered at high risk of severe infectious diseases.
“Infants die from many infections that adults don’t die from. The best thing we can do for this is vaccinate,” said Ramilo. “We know this strategy works, but we still don’t understand how the vaccines work very well.”
Published in Nature Communications, Ramilo’s team looked at responses to a routine vaccination series given to infants at 2 months of age. They found substantial heterogeneity in the babies’ antibody responses to the different components of the initial vaccination, in contrast to what has been observed in older children. The researchers took a systems-level approach to explore the immune response over time, using bulk and single-cell transcriptomics. This allowed the researchers to explore the response with the benefit of a true baseline.
The study found substantial heterogeneity in the antibody responses among the infants in the study. Single-cell transcriptomics offered the chance to identify the cause, which turned out to be the interferon response. Interferon functions as the cell’s alarm, recognizing invading pathogens and mobilizing the immune system. Given the centrality of interferon to both the innate and adaptive immune response, investigating these differences further may offer novel insights.
“We have begun to uncover these mechanisms and can think about how to design more effective vaccines for infants,” Ramilo concluded.
Once children reach a year old, vaccines are more consistently effective, though they can still be improved. Scientists at St. Jude are also investigating how to improve vaccines, especially the seasonal influenza vaccine, for older children and adults.
Scientists from St. Jude, including Schultz-Cherry, are part of the National Institute of Allergy and Infectious Diseases Collaborative Influenza Vaccine Innovation Centers (CIVIC) program. Published in Pathogens, a team from CIVIC showed that a computational method could design a vaccine with subtle mutations in viral proteins that then protected mice from influenza H5 viruses with different paired neuraminidase proteins. That increased efficacy and broad action are promising for the future improvement of the seasonal vaccine.
“Through our work CIVIC, we found that with some adjustments to the live-attenuated vaccine, including changing flu proteins such as hemagglutinin, adding the neuraminidase, or modifying the vaccine platform,” Schultz-Cherry said, “we can protect models of high-risk populations from getting infected.”

In the last few years, we have developed whole-pathogen sequencing to track potential organism spread in the institution, even preemptively trying to look before there is an outbreak situation.
Director, Clinical and Molecular Microbiology
A world of other pathogens beyond influenza
In addition to being a giant in influenza research, Webster worked with Elaine Tuomanen, MD, former Infectious Diseases chair and current member of Host-Microbe Interactions, to build the extensive infrastructure and expertise in infection biology at St. Jude. Over the decades, their efforts have sparked a wealth of research projects exploring other pathogens that threaten human health. In parallel, St. Jude has developed cutting-edge clinical diagnostic laboratories to protect patients directly.
“In the last few years, we have developed whole-pathogen sequencing to track potential organism spread in the institution, even preemptively trying to look before there is an outbreak situation,” said Randall Hayden, MD, Clinical and Molecular Microbiology director. He also serves as the Global Pathology and Laboratory Medicine Transversal program director, giving him unique insights into microbes that could be risky to patients.
Hayden and Hana Hakim, MD, Department of Infectious Diseases, Infection Prevention and Control medical director and a clinical investigator studying ways to prevent infections in St. Jude patients, collaborated on a gene-sequencing study reported in Clinical Infectious Diseases. They used genetic sequencing to track the spread of Clostridioides difficile, a bacterium that causes diarrhea and colitis, in pediatric oncology and bone marrow transplantation patients.
“We found that C. difficile transmission among St. Jude patients is very rare,” said Hakim. “And that the vast majority of recurrent C. difficile infections in the same patient are caused by the same strain of a previous infection that the patient had rather than the acquisition of a new strain.”
“What we are doing from an infection prevention standpoint is working,” Hayden added. “Other institutions could use our results as a benchmark.”
In addition to bacteria and viruses, fungal infections are a major concern.
Candida is a genus of yeast that includes several species that infect humans and is a common cause of life-threatening health care–associated bloodstream infections in the United States. “Candida parapsilosis is among the most common causes of Candida infections in pediatric and neonatal patients and is responsible for an increasing number of invasive Candida infections in many parts of the world,” said P. David Rogers, PharmD, PhD, Department of Pharmacy and Pharmaceutical Sciences chair, whose lab focuses on understanding the root cause of antifungal resistance. “In the last five to 10 years, we have seen an uptick globally in resistance to the common antifungal fluconazole among clinical isolates of C. parapsilosis”.
As C. parapsilosis becomes resistant to antifungals, therapeutic options become limited. Published in Clinical Microbiology and Infection, a report from Rogers’s lab described their efforts to elucidate the underlying mechanisms driving antifungal resistance of C. parapsilosis and learn how to predict, prevent, or overcome it. Led by the efforts of University of Tennessee Health Science Center College of Graduate Health Sciences student Laura Doorley, PhD, Rogers’s team found that mutations in TAC1, in addition to a well-known mutation in ERG11, drive high-level resistance, shifting conventional wisdom about fluconazole resistance in this species.
One common antifungal, fluconazole, targets a protein involved in sterol synthesis, encoded by the gene ERG11. A mutation in ERG11 can increase its expression, which will then increase the expression of other proteins known as drug transporters. These transporters will nonselectively pump antifungals out of fungi cells. In the study, mutated TAC1 increased the expression of several drug transporters to higher levels than mutated ERG11 alone, though both in combination drove the highest levels of antifungal resistance.
A legacy leading the future in pathogen research
From Webster’s initial work collecting influenza samples from wild waterfowl, the path paved by such infectious disease luminaries has been widening and expanding into what can today be considered a globe-spanning program based at St. Jude. By investing in understanding the viruses, bacteria, and fungi that pose a risk to patients, St. Jude is leading an entirely new generation of researchers to stay one step ahead of pathogens.
“I have to admit that I’ve been very spoiled by St. Jude,” Webster concluded. “The facilities here are top-class, letting us lead the international infectious disease research community. That’s only possible through the full support of WHO, NIAID, and St. Jude donors. I hope that support continues so my trainees can continue the fight against the flu and other pathogens.”