St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
Blinatumomab added to chemotherapy improves survival for adults with acute lymphoblastic leukemia

Charles Mullighan, MBBS (Hons), MSc, MD, member of the St. Jude Department of Pathology is co-senior author of a New England Journal of Medicine paper on adding blinatumomab to chemotherapy to treat adults with leukemia.
B-cell precursor acute lymphoblastic leukemia (B-ALL) has a cure rate exceeding 85% in children, but outcomes in adults are poor. A phase 3 clinical trial recently demonstrated that adding the immunotherapy drug blinatumomab to consolidation chemotherapy in adults with [BCR::ABL1-negative] B-ALL increased three-year survival from 68% to 85%. The study was restricted to patients with no measurable residual disease after receiving standard intensification before the modified consolidation therapy. Investigators from Mayo Clinic, St. Jude Children’s Research Hospital and Memorial Sloan Kettering Cancer Center led the collaborative clinical trial.
“Overall, the magnitude of benefit for patients we saw was very striking,” said co-senior author Charles Mullighan, MBBS (Hons), MSc, MD, member of the St. Jude Department of Pathology. “It worked so well that it led to an early stop to the trials so patients could cross over from the control to the treatment group. The results suggest blinatumomab incorporation into consolidation therapy should become the standard of care for adult BCR-ALL.”
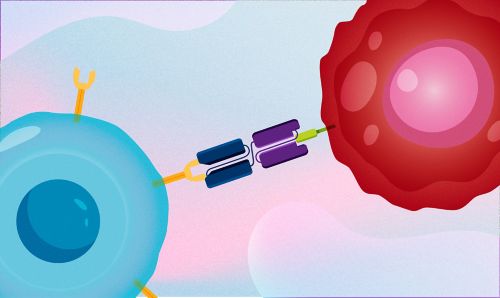
Blinatumomab draws together two separate molecules: CD3, a molecule on T cells responsible for tumor killing, and CD19, a protein present on cancerous B-cell precursors. Artwork by Briana Williams.
The study, published in the New England Journal of Medicine, tested the efficacy of blinatumomab, a drug that uses a patient’s own immune cells to target their cancer. The therapy draws together two separate molecules: CD3, a molecule on T cells responsible for tumor killing, and CD19, a protein present on cancerous B-cell precursors. Blinatumomab helps T cells find more tumor cells by bringing them together so the immune system can destroy leukemia cells. The drug is already approved for relapsed or refractory BCR-ALL.
While B-ALL therapies for adults have improved over the last decade, treatments for the disease in children have still performed better. Findings from the study showed blinatumomab significantly improved outcomes for adults with BCR::ABL1-negative B-ALL.
“B-ALL is an important cancer in adults. It previously did not have many effective therapeutic options, but this study shows the remarkable efficacy of blinatumomab,” Mullighan said.
Adding blinatumomab earlier in the treatment cycle
The study tested when it is best to give patients blinatumomab in the treatment process, moving it earlier than currently approved. During treatment, patients undergo an intensive regimen, followed by weeks of consolidation chemotherapy. The purpose of consolidation chemotherapy is to eliminate any cancer cells that might still be hiding in the body after the first initial treatment (called measurable residual disease). In the trial, only patients with no measurable residual disease continued to either receive consolidation therapy or consolidation therapy plus blinatumomab.
“The most exciting finding is that individuals who have no measurable residual disease, which has always been the group with the best chance of a cure, that those patients do even better if they’re treated with this immunotherapy,” Mullighan said.
The study suggests blinatumomab helps T cells find difficult-to-detect cancer cells that can linger after initial treatment, eventually giving rise to relapse if not treated.
Blinatumomab cuts across genetics and age
The study also included genetic sampling from each patient. That information then stratified the patients into favorable, intermediate, unfavorable and unknown risk groups. Previous studies have shown differences in treatment outcomes based on genetics, impacting risk group classifications. A benefit of blinatumomab treatment was observed in each genetic risk group. The study was not designed to determine the exact relationship between the therapy and patient genetics, but the universal results are promising.
“It was a bit surprising to see patients with genetically high-risk disease showing a favorable response,” Mullighan said. “In fact, we saw the activity of blinatumomab in all of the genetic risk groups, which is a promising observation that can then be further pursued in subsequent studies.”
In addition to cutting across genetic risk groups, the therapy promises to narrow the well-studied gap between childhood and adult B-ALL outcomes.
“We’ve grown used to seeing cure rates exceeding 90% in children,” Mullighan said, “but it’s been very different in the adult context. For the longest time, the cure rates of adult ALL were less than 50%. So, seeing response rates that are starting to approach childhood ALL is very exciting and encouraging for the future.”
The study’s first and corresponding author is Mark Litzow, Mayo Clinic. The other co-senior authors are Selina Luger, University of Pennsylvania Abramson Cancer Center and Martin Tallman, Memorial Sloan Kettering Cancer Center.






