St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
Rare childhood disorders widen window on diseases of aging
Through researching rare childhood disorders, Alessandro d-Azzo, PhD, found a resource of information on cellular biology and aging.
In her research, Alessandra d’Azzo, PhD, focuses on life-threatening inherited diseases of childhood. Few people have heard of these disorders because their incidence in the general population is very low.
Her short list includes sialidosis, galactosialidosis and GM1-gangliosidosis. These diseases belong to a group of about 70 genetic diseases called lysosomal storage disorders. Most are caused by mutations in a single enzyme or related protein that work in cellular compartments called lysosomes. The enzymes break down and recycle sugar-containing protein or lipids as well as other molecules in cells throughout the body. Without them, molecules accumulate and disrupt organ function, with devastating results.
“The disorders are rare, but I knew all along that these diseases could be a tremendous source of information on cell biology and aging,” said d’Azzo, endowed chair and member in the St. Jude Department of Genetics. Her confidence was strong in part because some lysosomal diseases have features of early aging. Understanding those mechanisms might improve understanding of neurodegenerative disorders, cancer and other diseases associated with aging.
But even d’Azzo has been surprised by where the research has led. “Never in my wildest imagination could I have anticipated that research on rare pediatric diseases could take me as far as it has, allowing me to branch out into different fields of biology with implications for common diseases of aging,” she said.
Those diseases include cancer, Alzheimer’s disease and, most recently, fibrosis. Fibrosis occurs when excess connective tissue accumulates in the muscles, lungs, liver, heart or other organs and disrupts their function. The cause is often unknown. Because connective tissue is produced in part by fibroblast cells, d’Azzo’s research led her to new questions.
Serendipity
d’Azzo began studying lysosomal storage disorders in the late 1970s. She was a postdoctoral fellow at Erasmus University in The Netherlands studying variants of GM1-gangliosidosis. This progressive, inherited disorder destroys neurons in the brain and spinal cord. GM1-gangliosidosis is caused by mutations in the gene GLB1, which has instructions for making the enzyme β-galactosidase.
“Serendipitously, I started to analyze patients’ fibroblasts that were considered GM1-gangliosidosis variants,” she said. “The research showed otherwise.”
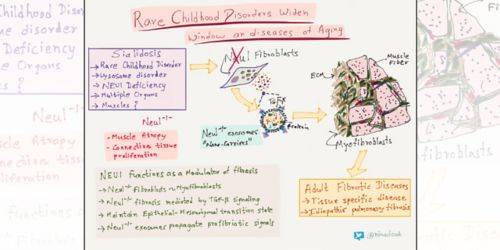
d’Azzo discovered that these variants carried mutations in the CTSA gene, which turned out to be the primary defect in another lysosomal disease, galactosialidosis. The findings redefined the disease. The CTSA mutations interfere with proper functioning of the lysosomal enzyme neuraminidase 1 (NEU1) and β-galactosidase. She went on to earn a second doctorate and develop a mouse model of galactosialidosis—one of the first models of lysosomal storage disorders.
Fibrosis
NEU1 enzyme deficiency due to mutations in the NEU1 gene causes the lysosome disorder sialidosis.
Here’s where serendipity re-enters the story.
Sialidosis causes a wide range of abnormalities in multiple organs, including muscles.
As a postdoctoral fellow in d’Azzo’s laboratory studying a mouse model of sialidosis, Edmar Zanoteli, MD, PhD, was intrigued by muscle changes. The connective tissue was expanding exponentially and relentlessly, invading the muscle fiber. He and Diantha van de Vlekkert, of d’Azzo’s laboratory, completed the first analysis of muscle degeneration in Neu1-deficient mice.
Zanoteli is now at the University of Sao Paulo Department of Neurology where he continues to research muscle disorders. Van de Vlekkert was recently first author on research from d’Azzo’s laboratory that appeared in Science Advances. The work detailed for the first time an association between NEU1 deficiency and fibrosis in humans and mice. “The research puts NEU1 deficiency squarely on the radar as a possible risk factor for development and progression of fibrotic diseases in adults for which the primary cause is unknown,” d’Azzo said.
She added: “The best discoveries in science come from serendipity.
“My great hope is that by finding processes that could be applicable to common adult conditions, it will also lead to treatments and even cures for children with these devastating rare disorders.”






