St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
Wilburn Reddick Lab
Analyzing brain structure via MRI to assess the impact of treatment on brain development and neurocognitive function
About the Reddick lab
A developing brain is a complex organ in a state of constant change. However, when an immature brain also receives treatment for catastrophic disease, normal developmental patterns can become disrupted. Additionally, multi-faceted treatments may damage existing brain structures, resulting in decreased neurocognitive performance. Our work aims to understand how such damage happens and what causes it, so we can identify targets for neurological interventions.

Our research summary
As we seek to advance the knowledge of structural brain changes that occur as a result of treatment for catastrophic pediatric disease, our work concentrates on five areas that expand what we know of connections within the human brain. To do this, we focus on understanding how these connections form networks that, when disrupted, interrupt key developmental patterns and lead to decreased neurocognitive outcomes.
Assessing changes in connectivity and executive brain networks
In patients with medulloblastoma, a spectrum of neurocognitive disorders can occur after completion of therapy, which include working memory deficits and associated spatial memory difficulties.
To better understand the connections and networks that influence working memory, we study the structural connectome of the human brain, which is observed when we parcellate the brain into anatomical regions.
Throughout our research, we have homed in on a specific subnetwork that is the working memory/executive function network. We use diffusion tracer imaging to generate tractography (directional tracks) to look at connections from each cortical parcellation to every other cortical parcellation. By doing this, we generate a matrix of 379 x 379, where we can see the connection strength for each of those intersections.
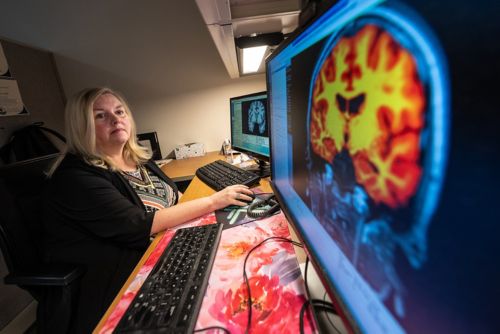
In patients with medulloblastoma who were enrolled in clinical trial SJMB12, we conducted diffusion tensor imaging on patients before and after the administration of radiation and chemotherapy and then at a designated follow-up point. Our work in this area is bolstered by a collaboration with Thomas Merchant, DO, PhD, to conduct an analysis of radiation dosimetry. We utilize individualized composite dosimetry maps, provided by Merchant, to discern what radiation dose was administered to each particular region. Having these composite dosimetry maps allows us to account for radiation dose effects, which vary by patient and risk arm.
This data allows us to assess brain connection changes in children ages three and up with medulloblastoma, the knowledge of which is being used to design the imaging protocol for upcoming medulloblastoma clinical trials.
Posterior fossa syndrome
Posterior fossa syndrome is defined by a collection of symptoms that occur following surgery. Through collaborations with Raja Khan, MD, and Heather Conklin, PhD, we are conducting a study in patients treated for medulloblastoma to assess connectivity changes between the thalamus and the cortex of the brain in response to surgery-causative damage in the cerebellum. We are specifically targeting cortices responsible for language, motor control, and somatosensory perception.
Our aim is to understand how brain connectivity in patients who experience posterior fossa syndrome differs from other patients who receive similar surgeries.
Survivorship assessment
We hold several collaborations with investigators in the Departments of Psychology and Behavioral Sciences and Epidemiology and Cancer Control to aid in the detection of structural brain changes in survivors of childhood cancer.
In the area of Hodgkin lymphoma, we collaborate with Kevin Krull, PhD, to examine changes in the brain structure that occur as a result of distant radiation in the body. The distant radiation can cause vascular changes that lead to modified neurocognitive networks.
In a collaboration with Tara Brinkman, PhD, we study acute lymphoblastic leukemia (ALL) survivor cohorts who received a neurostimulation intervention to offset neurocognitive effects. Our work in this area assesses changes in the underlying structural networks that use the structural connectome.
In a collaborative effort with Nicholas Phillips, MD, PhD, we work to correlate hypothalamic volumes with endocrine and neurocognitive outcomes in survivors of medulloblastoma.
All our collaborative efforts in this area aim to improve outcomes for survivors of childhood cancer.
Fusing structural connectome to the functional connectome
A study of structure is incomplete without understanding its functional counterparts. In collaboration with Raganatha Sitaram, ME, PhD, we seek to fuse the structural and functional together to learn how one informs the other. Using functional connectome data captured from functional magnetic resonance imaging (fMRI) in Sitaram’s lab, we infuse our structural connectome data to create an overall view and analysis of what’s happening in the brain, which additionally allows us to identify targets for future neurological interventions.
Contributing to mediational models with a high-dimensional approach
In a project led by collaborative efforts with Yimei Li, PhD, and Cai Li, PhD, data scientists at St. Jude are working to build statistical models that are able to utilize high-dimensional data from brain imaging as a mediator between therapy and neurocognitive outcomes. By developing this mediational model with our high-dimensional imaging data, we hope to give researchers and clinicians the ability to process and utilize data acquired with advanced imaging techniques.
At the core of our efforts lies our desire to characterize the connections and networks of a developing brain receiving treatment for catastrophic diseases. By doing this, we may be better able to contribute to future therapies and interventions that offset developmental delays and neurocognitive effects in children with catastrophic diseases.
Publications
Contact us
Wilburn Reddick, PhD
Member, St. Jude Faculty
Department of Radiology
MS220, Room I3108
St. Jude Children's Research Hospital
Follow Us

Memphis, TN, 38105-3678 USA GET DIRECTIONS