St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Vector Laboratory (SJVL)
Advancing innovative viral vector technology for research and treatment of catastrophic pediatric diseases
Our laboratory is devoted to facilitating the use of viral vector systems at St. Jude. We have significant expertise and experience with the following viral vector systems:
- Adeno-associated viral vectors (serotypes 1, 2, 5, 6, 8 and 9)
- Retroviral vectors
- Lentiviral vectors
Vector development by the numbers
Developed suspension cell line grown in serum-free media for production of clinical grade LV.
Vector development by the numbers
Significant cost savings for St. Jude investigators compared to outside providers (depending on source and other details cost savings can be as low as $400,000 or as high as $10,000,000).
Vector development by the numbers
Developed Virus-like Particle packaging Cas9-sgRNA for editing primary T cells (expected to save >$500,000 per target compared to traditional Cas9-RNP)
Vector development by the numbers
Produces over 1,000 vector preparations in support of St. Jude investigators each year.
OVERVIEW
The Vector Development and Production Shared Resource supports viral vector-related research at St. Jude with dedicated, separate laboratory space for DNA construction and virus production and an archive of well-characterized cellular and plasmid reagents. The lab assists St. Jude investigators by providing both small-scale and large-scale vector production services for lentiviral, gamma-retroviral and Adeno-associated viral vectors.


IMPACT
A portion of our laboratory efforts are devoted to assisting researchers in the production of viral vector technology at St. Jude. We accomplish this in several ways, the simplest of which is the provision of validated plasmid reagents which can be transfected into SJ293TS-DPB cells for production of virus or used for the insertion of genes to make new, transfectable vector plasmids.
EQUIPMENT
- (2) Cytiva Akta Avants (vector purification)
- (2) Beckman Coulter ultracentrifuges (vector concentration/purification)
- Bio-Rad Autodg ddPCR system with two readers (titration/vector copy number determination)
- Eppendorf epMotion 5075vt liquid handling robot (titration)
- (2) Oxford Nanopore Minion (DNA/RNA sequencing)
- (3) N-BioTek shaking incubators (culturing suspension cells for vector production).
SERVICES
- AAV, LV, & γRV Vector Requests
- LV & γRV Production and Titration
- AAV Production and Titration
- Distributed Vector & Helper Plasmids
- Recommended Protocols & Reagents
- Vector Delivery and Charge Back to Cost Center
Director

Robert Throm, PhD
Director
robert.throm@stjude.org
Dr. Robert E. Throm is the Director of the Vector Laboratory at St. Jude. He earned a Ph.D. from the Indiana University School of Medicine with a specialization in Microbiology and Immunology, complemented by a Bachelor of Science in Biology from Indiana University. Since joining St. Jude Children's Research Hospital in 2001 Dr. Throm has been instrumental in advancing vector development, progressively assuming roles such as Senior Research Technologist and Scientific Manager, Clinical Vector Development, before becoming the Director in 2019. His pioneering work in designing and producing novel lentiviral and Adeno-associated viral vectors, including a revolutionary concatemeric array transfection technique, has significantly contributed to the vector field. Dr. Throm's dedication to biosafety and commitment to scientific progress position him as an invaluable asset to both St. Jude and the wider scientific community.
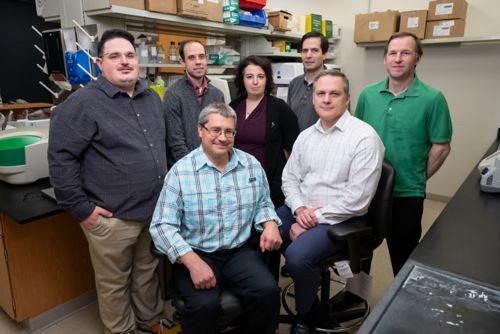
Meet the team
Clockwise from back row, left:
Jordan Beard, PhD, Lead Researcher
Brandon Lowe, PhD, Manager of Lab Operations
Francesca Ferrara, PhD, Scientist
Christopher Wincek, Lead Researcher
Matt Bauler, Lead Researcher
Robert Throm, PhD, Director
Matthew Wielgosz, PhD, Sr. Scientist-Managing.