St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
New JAK PROTACs (SJ-21-0010)
St. Jude Reference #SJ-21-0010
Researchers at St. Jude have developed small molecule conjugates of JAK1/JAK2 inhibitors ruxolitinib, baricitinib and cereblon binders including known immunomodulatory drugs (IMiDs) and novel cereblon binder. The invention can be used for development of the treatment of tumors driven by aberrant JAK-STAT signaling, including CRLF2-rearranged (CRLF2r) ALL. CRLF2-rearranged ALL comprises up to 60% of Philadelphia-like (Ph-like, BCR ABL1-like) acute lymphoblastic leukemia (ALL), and up to 15% of B-ALL overall, and is associated with high-risk features and poor outcome. Targeting of this pathway with type I JAK inhibitors such as ruxolitinib results in variable inhibition of signaling and proliferation in vivo, and emerging clinical data indicate suboptimal responses to ruxolitinib. Thus, new approaches to abrogate JAK-STAT signaling are urgently required to improve the dismal outcomes for CRLF2r ALL patients and may have broader therapeutic application in other JAK-STAT driven malignancies.
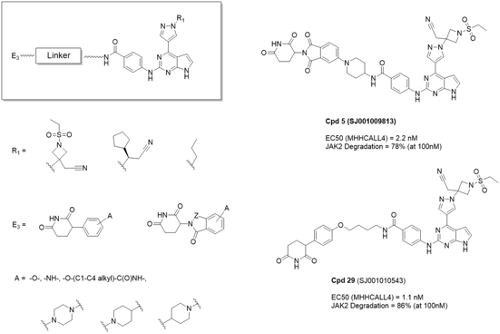
Their novel ruxolitinib and baricitinib-CRBN conjugates were designed and synthesized utilizing both novel CRBN binders (e.g. PG: Phenyl Glutarimide) discovered in-house and known IMiDs with unique linker components including piperazine and piperidine. They showed potent and selective JAK2 degradation activities with unprecedented in vitro potency in human ALL cell lines. For example, baricitinib-PG conjugate, compound 5 (SJ001009813, Fig. 1), displayed around 78% JAK2 degradation while only 29% degradation for GSPT1 which has been known as one of the main off-target of conventional CRBN based PROTACs. In addition, this compound 3 showed over 2,500 times higher efficacy in human CRLF2r cell lines (MHHCALL4 EC50= 2.2 nM) than ruxolitinib and baricitinib (12 mM and 5 mM, respectively). Compound 29 (SJ001010543, Fig. 1), exhibited 86% JAK2 degradation with limited degradation (~49%) of GSPT1. Compound 29 also showed potent cellular activity in human CRLF2r cell lines (MHHCALL4 EC50= 1.1 nM).
Keywords
Small molecule, JAK1/JAK2 inhibitor binders, CRLF2r and JAK-STAT driven ALL, Ruxolitinib, Creblon, Baricitinib.
Granted Patents or Published Applications
A provisional patent application has been filed.
Related Scientific References
Yunchao Chang, Jaeki Min, Jamie A. Jarusiewicz, Marisa Actis, Shanshan Yu-Chen Bradford, Anand Mayasundari, Lei Yang, Divyabharathi Chepyala, Lisa J. Alcock, Kathryn G. Roberts, Stanley Nithianantham, Dylan Maxwell, Lauren Rowland, Randolph Larsen, Aman Seth, Hiroaki Goto, Toshihiko Imamura, Koshi Akahane, Baranda S. Hansen, Shondra M. Pruett-Miller, Elisabeth M. Paietta, Mark R. Litzow, Chunxu Qu, Jun J. Yang, Marcus Fischer, Zoran Rankovic, Charles G. Mullighan; Degradation of Janus kinases in CRLF2-rearranged acute lymphoblastic leukemia;” Blood (2021) 138 (23): 2313–2326. Published online, June 10, 2021
Licensing Opportunities
We are seeking a partner who would develop this technology and commercialize and offer the resulting drugs.
Contact the Office of Technology Licensing (Phone: 901-595-2342, Fax: 901-595-3148) for more information.