St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
Combination IFN and NEI therapy for cancer (SJ-21-0033)
St. Jude Reference #SJ-21-0033
Description
Cancers are a leading cause of death around the world, and treatment strategies for many cancers remain limited. One of the key hallmarks of cancer is the ability of tumor cells to resist cell death. Harnessing the power of the body’s innate immune system to activate cell death pathways therefore represents a tempting therapeutic strategy.
Researchers at St. Jude have investigated programmed cell death pathways to identify and understand regulatory mechanisms. These studies led to the identification of the innate immune sensor ZBP1 and the related protein ADAR1 as interacting partners that regulate inflammatory cell death and tumor growth.
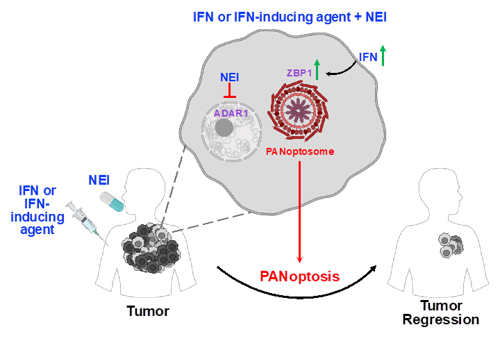
ZBP1 is an interferon (IFN)-inducible innate immune sensor that activates an inflammatory cell death pathway known as PANoptosis. PANoptosis integrates elements of other cell death pathways, such as pyroptosis, apoptosis and necroptosis. ADAR1’s function in cell death and PANoptosis was previously unclear, although mutations in the protein are associated with human cancers and several autoimmune and autoinflammatory disorders. The researchers showed how ADAR1 bound to and suppressed ZBP1 activity in the cytoplasm, prevented PANoptosis, and inhibited cell death. Sequestering ADAR1 in the nucleus through the use of nuclear export inhibitors (NEIs) freed ZBP1 to trigger PANoptosis, inflammatory cell death, and regress tumors.
Researchers at St. Jude have invented a therapeutic combination with IFN-γ and the FDA-approved NEI KPT-330 which disrupted the ADAR1-ZBP1 interaction and induced robust cell death. These agents together significantly regress melanoma tumors in mice. The mechanisms identified here explain the reasons behind the failure of IFN therapy in cancer and suggest that combining IFN with NEIs can be used for the treatment of cancer and will improve treatment outcomes. Additional formulations could include the combination of one or more IFNs, including IFN-α, IFN-β, and/or IFN-γ, or IFN-inducing agents with one or more NEIs, including KPT-330, leptomycin, and/or others.
This invention can be used to identify and develop therapeutics to induce cancer cell death and reduce or eliminate tumor burden, or to prevent or cure autoinflammatory diseases driven by IFNs.
Keywords
Combination IFN and NEI Therapy, Cancer, interferons (IFNs), nuclear export inhibitors (NEIs), cell death, KPT-330, leptomycin B, PANoptosis, RIPK3, ZBP1, ADAR1, drug discovery and development
Granted patents or published applications
Application filed, available under confidentiality
Related scientific references
Press release about the scientific article: https://www.stjude.org/media-resources/news-releases/2021-medicine-science-news/promising-preclinical-cancer-therapy-harnesses-a-newly-discovered-cell-death-pathway.html
Karki R, Sundaram B, Sharma BR, Lee S, Malireddi RKS, Nguyen LN, Christgen S, Zheng M, Wang Y, Samir P, Neale G, Vogel P, Kanneganti TD. ADAR1 restricts ZBP1-mediated immune response and PANoptosis to promote tumorigenesis. Cell Reports. 2021. 37(3):109858.
Licensing opportunities
We are seeking partners to develop therapies/therapeutics using this new method for treating cancer by inducing cancer cell death through combinations of IFNs or IFN-inducing agents and NEIs, or to prevent or cure autoinflammatory diseases driven by IFNs.
Contact the Office of Technology Licensing (Phone: 901-595-2342, Fax: 901-595-3148) for more information.