St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
Successful trials result in a commercial partner for XSCID treatment
X-linked severe combined immunodeficiency (XSCID, or ‘bubble boy disease’) is a rare genetic disorder that occurs in 1 to 2 births per 100,000. The children are born lacking the ability to produce T cells or natural killer (NK) cells; and although they have a normal number of B cells, they are not functional. As a result, XSCID patients often suffer from potentially deadly bacterial, viral, or fungal infections very early in life.
Various XSCID gene therapy clinical trials have been conducted over 20 years, both as an alternative to hematopoietic stem cell transplant (HSCT) or following a poor outcome. Transduction of normal copies of the mutated IL2RG gene into stem cells using a gamma-retroviral vector has previously resulted in reconstitution of patient NK and T cells, but not functional B cells; and early trials resulted in vector-induced leukemia in 25% of patients due to insertion of the gene-carrying retrovirus near an oncogene.
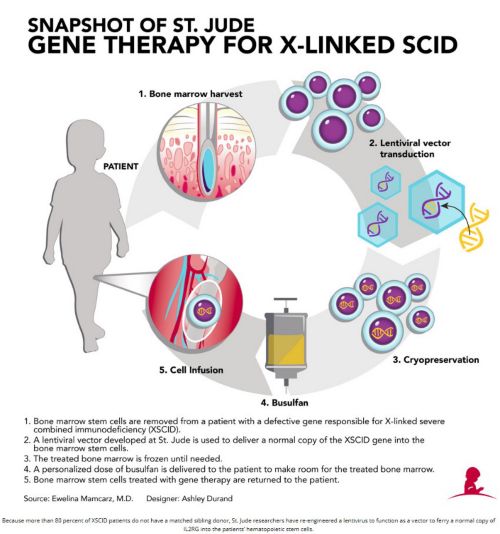
Historically, the most effective treatment for XSCID has been bone marrow transplantation, [i.e. hematopoietic stem cell transplantation (HSCT)], where a patient receives healthy blood-forming cells from a matched sibling donor, or a half-matched parental donor. Physicians have also had success using umbilical cord blood, which is rich in stem cells. Overall survival for the 10% of patients who have matched sibling donors is 95%; however, it falls to 60 to 75% with other types of transplant, with over 50% of these patients requiring lifelong intravenous immunoglobulin therapy. In addition, 28% experience acute graft-vs-host disease (GvHD), and 15% experience chronic GVHD. T-cell immunity may also decrease over time, requiring 26% of patients to undergo a second HSCT. The earlier age the treatment is provided, the better the clinical outcome, so now all 50 U.S. states and many countries screen newborns for XSCID. Above is the St. Jude procedure to reconstitute the immune system.
A lentiviral vector from St. Jude Children’s Research Hospital was investigated in multicenter clinical trials in conjunction with reduced-exposure busulfan conditioning. The vector was made on our campus by our GMP facility that produces clinical grade biological products, which also re-engineers the patients’ cells to carry the healthy new gene.
In the first trial, reported in the April 2016 issue of Science Translational Medicine, five males aged 10 to 23 years with progressively declining persistent immune dysfunction after parental HSCT in infancy were treated at the National Institute of Allergy and Infectious Diseases (NIAID). Patients all had chronic viral infections and other XSCID related health problems after HSCTs failed to fully correct their immune function. Previously, patients on gene therapy trials gained T cells; but still lacked B cells, NK cells, and antibodies; requiring lifelong gamma globulin therapy. Now, more than 2 years after undergoing this new gene therapy, initial patients are producing a greater percentage of immune cells, including T, B and natural killer cells.
The clinical trial included a number of firsts for gene therapy of XSCID:
- The first use of a lentiviral vector
- A streamlined stable production system (increases supply)
- Novel features designed to increase safety/effectiveness
- Busulfan used to re-establish the gene-corrected stem cells
Results for 8 patients under the age of 2 with XSCID in the second trial were presented at the 21st Annual Meeting of the American Society of Gene & Cell Therapy in May 2018. Six patients achieved reconstituted immune systems within 4 months following treatment, with 2 of the 6 patients discontinuing monthly infusions of intravenous immunoglobulin. The remaining patients, at earlier stages of recovery, continue to progress favorably. All infections resolved in the 3 patients who had disseminated infections prior to therapy.
The trials continue, with those under the age of 2 being treated at St. Jude, UCSF Benioff Children’s Hospital, and Seattle Children’s Hospital; and older patients being treated at the NIH. Two older patients demonstrated immune system reconstitution and clinical improvement at 2 to 3 years following treatment. In 3 younger patients, similar levels of gene-modified immune cells were also observed at 6 to 9 months following treatment. In 2018, these trials resulted in an exclusive worldwide license agreement between St. Jude and Mustang Bio to further develop and commercialize this therapy.

Samuel was one of 6 patients who achieved reconstituted immune systems within 4 months after gene therapy. He received his last immunoglobulin infusion in January 2018. At 16 months, Samuel returned home to Brazil in January 2018 with no activity restrictions, thought his parents will bring Samuel back to St. Jude for checkups. To read more about this discovery and Samuels' personal story regarding its impact, go to: https//www.stjude.org/scid-second-chance.