St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude's Contribution to chimeric antigen receptors (CARs) with 4-1BB stimulatory signaling domain
The use of cell therapy and immunomodulation has become a hot area of study and development which has resulted in the translation of academic research into clinical therapies for B-cell malignancies.
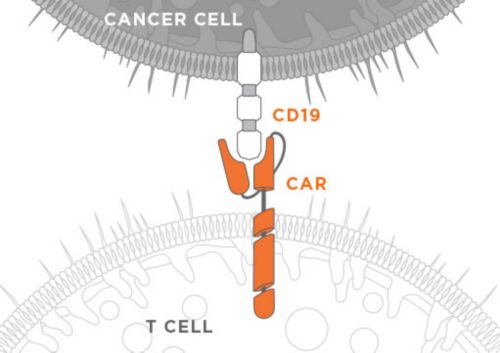
In the early 2000s, Dario Campana, MD, PhD, and Chihaya Imai, MD, PhD, developed a chimeric antigen receptor (CAR) to be expressed in an immune cell to recognize the CD19 antigen, which is prevalent on the B cells that cause acute lymphoblastic leukemia (ALL), B-cell chronic lymphocytic leukemia (CLL) and B-cell non-Hodgkin lymphoma (NHL). One CAR made at St. Jude was composed of an anti-CD19 single chain variable fragment extracellular domain, a CD8 alpha transmembrane domain, and a cytoplasmic domain containing 4-1BB and CD3zeta signaling domains. Anti-CD19 CAR immunotherapy works by genetically modifying a patient’s immune cells to express the CAR protein, which in turn stimulates these cells to attack and kill CD19 antigen expressing cancer cells.
The Office of Technology Licensing (OTL) at St. Jude Children’s Research Hospital filed a patent application in 2003 based on Campana and Imai’s work, claiming, among other things, compositions for genetically modifying human immune cells to target and destroy cancer cells. Campana publicly related findings at a conference later in 2003. This led to further research and collaboration and eventually to the testing of CAR modified T-cells in clinical trials that garnered international attention.
Today, multiple therapies that utilize Campana and Imai’s patented CAR are showing promising clinical results in trials at numerous institutions in the U. S. and abroad – many with commercial sponsors. Results associated with these trials have shown such promise that Science labeled cancer immunotherapy as the breakthrough of the year in its December 20, 2013 issue, and both progress and commercial funding have accelerated. Startups like Juno Therapeutics and Kite Therapeutics, as well as larger organizations like Novartis, aim to have an approved CAR T-cell treatment on the market soon.
A St. Jude’s patent related to Campana and Imai’s work was issued in March of 2013. Later that same year St. Jude exclusively licensed this patent and other related patent rights to Juno Therapeutics. Juno aggregated these patent rights with technology and rights from a number of entities, including Fred Hutchinson Cancer Research Center, Memorial Sloan Kettering Cancer Center and Seattle Children’s Research Institute, and has now raised over $300 million for further research, development and commercialization of immunotherapies. Juno intends to improve and leverage its cell-based platform to develop new product candidates that address a broader range of cancers and human diseases. Juno also announced a sublicense of certain commercial rights outside North America and China as part of a collaboration with a large biopharmaceutical company and have also announced the founding of a new company to leverage Juno’s CAR and other technologies in China.
Updates
In 2017, the FDA approved a Novartis drug based on the St. Jude CAR, called “Kymriah” for use in children and young adults with r/r B-cell ALL: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm574058.htm
In 2018, Kymriah was approved for treating adults with r/r large B-cell lymphoma: https://uk.reuters.com/article/novartis-pharmaceuticals/novartis-says-kymriah-receives-second-u-s-fda-approval-idUKL3N1S842O
JCAR017, the CD-19 CAR being developed by Juno Therapeutics/Celgene Corporation, is currently in clinical development. Celgene hopes to submit a biologics license application (BLA) in the second half of 2019. Celgene and Bristol-Myers Squibb have entered into a merger agreement, which is expected to complete in the second half of 2019.