St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
Capitalizing on the resolution revolution in structural and cell biology with cryo-electron tomography

Scientists at St. Jude are leveraging Cryo-ET capabilities for research.
You might have heard the famous axiom, “form follows function.” It’s a principle that applies to architecture as much as biology. Studying the shape and structure of biomolecules, such as proteins and nucleic acids, has always been essential to understanding how these molecules function. James Watson, Francis Crick and Rosalind Franklin famously built a picture of the structure of DNA that, in turn, helped us understand how genetic information is stored and transmitted during cell division.
However, visualizing the shape and structure of tiny objects like strands of DNA requires microscopy techniques that don’t rely on visible light. Many structures inside our cells are smaller than wavelengths of light in the visible spectrum, meaning that optical microscopy techniques can’t resolve their fine details.
Researchers discovered DNA’s structure using X-ray crystallography, which measures how an X-ray beam scatters as it passes through a crystallized sample. X-ray crystallography, developed in the early 1900s, was among the first techniques to overcome light microscopy’s resolution limitations. However, it doesn’t work well for large complex biological molecules like proteins, which may not readily form crystals. Moreover, researchers need to study these molecules’ dynamic structures and shapes in the context of where they do their work — inside living cells.
At St. Jude Children’s Research Hospital, Syed Asfarul Haque, PhD, Director of the Cryo-Electron Microscopy Center in the Department of Structural Biology, lights up when he talks about the Center. It started with a vision that any researcher at St. Jude—of any experience level—could take advantage of the revolutionary capabilities of cryo-electron microscopy (cryo-EM) and now cryo-electron tomography (cryo-ET). These specialized imaging techniques use electron beams, with far more resolving power than light, to visualize complex frozen biological structures at incredible resolutions—to the point of measuring the distance between individual atoms within said molecules. They are opening up new possibilities for seeing tiny cellular components in action.
Providing expertise through a central resource
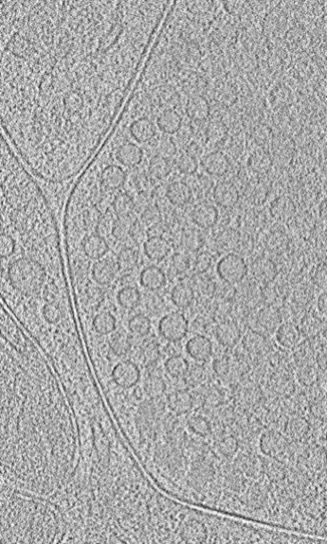
A single slice from a 3D-reconstructed Cryo-ET visual of vesicles (round circular structures approximately 40 nanometers in diameter) that store neurotransmitters.
In 2017, the Nobel Prize in Chemistry was awarded for cryo-EM's development. This imaging technique started a “resolution revolution,” allowing researchers to see biological structures in finer detail. Using a “flash” freezing approach to prepare samples for electron imaging, scientists can observe how proteins move, how small-molecule drugs bind to their targets and where and how different compartments within a cell touch and interact. However, as a relatively new and expensive technique that produces massive datasets, cryo-EM can be daunting to researchers without prior experience. The St. Jude Cryo-EM Center is changing that — it democratizes cryo-EM resources and supports users with training and data analysis.
“Microscopy and core equipment centers at other institutions often charge researchers for microscope time, and researchers are often left to figure out how to process and interpret the data,” Haque said. “We do things differently. We cover all expenses and guide researchers through preparing samples and processing data. We also train researchers to become independent in their cryo-EM skills.”
The Center houses several electron microscopes, including a new microscope with a cold field emission source called the Titan Krios G4, among the world’s highest-resolution setups. Using these microscopes, St. Jude researchers are looking more closely at a host of cellular structures, learning about the cellular machinery involved in degrading cancer-causing proteins, groups of interacting proteins such as the Rab29—LRRK2 complex involved in Parkinson’s disease, small-molecule drugs that might serve as new antibiotics, virus-like particles and more.
“The exchange of knowledge is key to scientific innovation,” says Martin Turk, PhD, a senior scientist in the Department of Structural Biology. The scientists who work in the Cryo-EM Center, including Yuewen Sheng, PhD, José Miguel de la Rosa Trevín, PhD and Sagar Chittori, PhD, provide consultation, hands-on support, mentoring, computing resources and training to guide researchers through complex electron microscopy workflows.

A microchip that Imoto’s group uses to stimulate and freeze brain tissue samples for cryo-ET.
The power of native 3D imaging
The Cryo-EM Center’s newest capability is cryo-electron tomography (cryo-ET), which constructs 3D images of intact biological structures by combining many views, taken from multiple angles, of a frozen sample. The sample might be a protein complex, an individual cell, or even a thinly sliced whole piece of tissue. Scientists can see proteins and other molecules in their natural context using cryo-ET. It is a game-changing technology for understanding complex cellular components and their interactions.
Imagine you are a wildlife biologist studying the behaviors of highly social animals, such as wolves or dolphins. A significant difference exists between studying these animals in captivity — in a zoo or an aquarium — versus observing them in their natural habitat. They travel massive distances in the wild and have complex social interactions. Studying them only in a zoo or aquarium’s controlled and limited environment, you might miss or even misconstrue critical aspects of these animals’ native behaviors.
The behaviors of biological components such as proteins, membranes, and other biomolecules are similarly complex and dynamic within the environment of a living cell. Before the development of cryo-ET, these biological components usually had to be extracted, purified and processed for imaging. An unintended consequence of the preparation process was that researchers couldn’t see what these components looked like while interacting with their natural cellular environment. But with cryo-ET, researchers can flash freeze a living tissue, then slice it and obtain thousands of snapshots to reveal dynamic interactions between cells, membranes, proteins, and more.
Yuuta Imoto, PhD, Department of Developmental Neurobiology, is studying how and why human brain cells can communicate so much more rapidly than the nerve cells of animals such as jellyfish. Neurons in our brains communicate through small molecules called neurotransmitters that travel between neurons. These neurotransmitters are stored and released by membrane-bound compartments called synaptic vesicles. To pull your hand away from a hot stove, for example, hundreds of these vesicles release neurotransmitters in less than a second. Neurons need to be able to quickly recycle the vesicles — absorbing used ones and creating new ones — to sustain rapid communication.
Researchers have debated how vesicles can be recycled so quickly. Some have argued that the neurons recycle and create new vesicles through endocytosis too rapidly for proteins to control the process. But Imoto thought the responsible proteins just hadn’t been seen in action yet.
Imoto and his lab are now combining cryo-ET and a time-resolved electronic microscopy technique to build 3D snapshots of the rapid recycling of vesicles. The researchers can send an electric current through a piece of brain tissue using special microchips. After just milliseconds of electric stimulation, they can rapidly freeze all the neurons in that tissue at a rate of 25,000°C per second. Using cryo-ET to visualize these frozen samples, Imoto and his group are able to see vesicles forming and releasing neurotransmitters, the proteins involved and how these proteins organize in a 3-dimensional space.
Imoto’s group recently observed that a protein called dynamin (Dyn1xA) accelerates the creation of synaptic vesicles by neurons. Humans have a special fast-acting form of this protein that jellyfish don’t have. Mutations in Dyn1xA might also be responsible for a slowing neuronal communication in various cognitive disorders.
“Our lab was able to see structures that have eluded researchers for decades because endocytosis is too fast to capture by traditional methods,” Imoto said. “Cryo-ET and time-resolved EM freezes it in time, letting us view the native machinery in action and at high resolution.”
Cryo-EM and cryo-ET are helping Imoto’s lab push the boundaries of neurobiology research, offering glimpses into the structural dynamics of neurons and synaptic vesicles in near-native conditions.
That’s not to say that using cryo-ET to get high-resolution snapshots of specific elements within intact, flash-frozen tissue is easy. This power of cryo-ET — being able to visualize the inner workings of cells in their native complexity versus purifying and look at single proteins “out of context” — is also its greatest challenge.
Where’s Waldo?

Elizabeth Kellogg, PhD, looks in on the inner workings of the Titan Krios G4 microscope.
“The beauty and horror of Cryo-ET is that you see absolutely everything in the cell,” says St. Jude researcher Elizabeth Kellogg, PhD, Department of Structural Biology. “You can find things nobody knew existed because they weren’t explicitly labeled. At the same time, the technology is still so new that every step of a cryo-ET experiment requires optimization and is prone to failure. We’re at the frontier of what can be done.”
Kellogg and others in her lab are using cryo-ET to study protein complexes (multiple proteins interacting) that transcribe the instructions in the genetic code into messenger RNA. These proteins have been imaged in isolation but never visualized in their active conformations inside cells. Tharun Chand, a researcher in Kellogg’s lab, equates finding these complexes within a flash-frozen cell using cryo-ET to finding a single person in an aerial photograph of Manhattan. Even using fluorescent protein tags to narrow in on the area of interest, the task is akin to a giant “Where’s Waldo” puzzle.
Getting a 3D cryo-ET image of a specific protein complex of interest within a frozen cell’s crowded and chaotic environment is nearly impossible. But Kellogg and Chand are making it possible using fruit fly salivary glands. These glands have the unique feature of polytene chromosomes, giant bundles of DNA formed by repeated copies of the same DNA sections. Zooming in on these chromosomes reveals thousands of copies of the transcription protein complexes Kellogg’s lab is interested in.
By combining specialized model organisms with the resolution power of cryo-ET, Kellogg and colleagues are working to visualize the atom-level morphology of protein complexes that transcribe our DNA into RNA at an unprecedented level of detail.
With the capabilities of the St. Jude Cryo-EM Center, other researchers across the institution will also be able to harness these groundbreaking imaging techniques to make discoveries in structural and cell biology.
"St. Jude is really putting the pieces together to make this one of the hottest sites for cryo-ET and structural biology in the U.S.,” Kellogg said.






