St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
Drug which blocks stress granule formation offers insight into biomolecular condensates
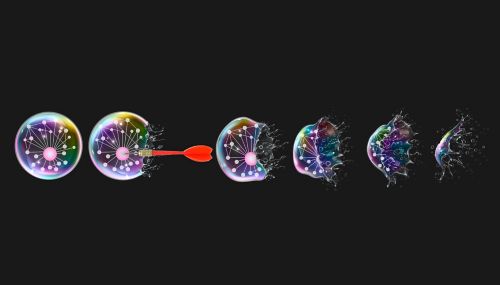
Novel inhibitors which bind to G3BP1, the central node of stress granules, are shown to effectively inhibit condensate formation, offering route to further study their biology and link to neurodegenerative disease pathology. Artwork by Leena Xaypanya.
Drug design can be as simple as identifying a molecule that hits a target and optimizing it for effectiveness and safety. However, complex cases require more creative solutions. Scientists often need to think outside the box, creating therapies that hit specific pathways with downstream effects on a disease-causing entity. In these cases, understanding the underlying processes that lead to disease is vital. In the most difficult of situations, this understanding can lead to exceptionally creative therapeutic breakthroughs.
In the last fifteen years, there has been an explosion of interest in the biomedical research community in biomolecular condensates, temporary compartments in cells that can form droplets, like oil in water. They contain high concentrations of biomolecules such as proteins, RNA, and DNA. Importantly, their existence is transient, forming and dissolving in the cell as needed. These droplets organize biomolecules at specific locations, increase reaction rates and compartmentalize biomolecules selectively to regulate signaling.
Today, condensates have shown the potential to be paradigm-shifting therapeutic targets. However, their link to disease remains a puzzle that researchers at St. Jude are working to solve.
The link between condensates and disease
Research into biomolecular condensates is in a golden era, as more and more cellular processes are postulated to be driven or supported by their unique properties. This also means they are never far from disease, with evidence showing dysregulation of their formation and dissolution to have pathogenic consequences.
One such group of condensates, ribonucleoprotein (RNP) granules, are linked to neurodegenerative diseases such as amyotrophic lateral sclerosis, and frontotemporal dementia, as well as cancer. While RNP granules have numerous functions, under stress conditions, a specialized RNP granule called a stress granule forms.
Cell stress, like viral infection or nutrient deprivation, leads to an intentional pause in translation, the protein assembly process. During this time, stress granules function as temporary storage units for the stalled messenger RNA until the stress is relieved and translation can be restarted.
Issues arise when, for one reason or another, these condensates fail to dissolve.
As part of the Collaborative Research Consortium on the Biology and Biophysics of RNP Granules, St. Jude researchers are investigating if directly targeting the formation of stress granules and other disease-linked condensates is a viable route to therapy.
New inhibitors allow for rigorous testing of stress granules
Recent work from the laboratory of J. Paul Taylor, MD, PhD, St. Jude Scientific Director and Department of Cell and Molecular Biology chair, published in the Journal of Cell Biology, demonstrated the ability of two molecules to inhibit the formation of stress granules selectively.
These inhibitors work by binding the hub protein of stress granules, G3BP1 and its paralog G3BP2. The inhibitors were developed by structure-guided design and built on a large amount of previous research from the Taylor lab into stress granule formation. In a 2020 publication in Cell, the team revealed that despite containing hundreds of different types of proteins, G3BP proteins function as the key central node, or cornerstone, of stress granules. Moreover, a detailed investigation of G3BP1 revealed an Achilles heel in one interface on the surface of the protein.
This present work represents a crucial step forward in investigating the viability of drugging condensates.
“These inhibitors are a tool to understand what these G3BP1 interactions and the stress granule as a whole do in cells,” said first author Brian Freibaum, PhD, St. Jude Department of Cell and Molecular Biology.
Viral replication offers unlikely path to drug discovery
Despite the significance of G3BP1, identifying molecules that can specifically bind to it has been arduous, with the protein being labeled “undruggable.” However, unrelated research into viral replication happened to identify G3BP1 as an important regulator of the replication process, and with this finding came a breakthrough.
“This drug screen actually began with what we learned from Chikungunya virus research,” said co-author Hong Joo Kim, PhD, St. Jude Department of Cell and Molecular Biology. “The virus expresses a protein called nsP3 that binds to G3BP1 and blocks the formation of stress granules.”
This revelation provided a starting point for drug development to begin in earnest. The researchers explored the binding mechanism of the short strand, or peptide, of nsP3 responsible for binding and re-engineered it. “We chopped and chopped and made a list of peptides that could block the formation of stress granules,” explained Kim. From this screen came two small molecules that mimicked the interaction of the nsP3 peptide with G3BP1, named G3Ia and G3Ib.
“Using live-cell imaging in our model system where G3BP1 is visualized, we confirmed that these molecules do inhibit stress granule formation,” said Freibaum. “It’s rapid too, with 90% of granules gone 30 seconds after adding the inhibitor.”
New route to tackle long-standing questions
The design of these inhibitors has huge implications for stress granule research and biomolecular condensate research at large. “The only way we could investigate G3BP function before was to delete the G3BP1 and G3BP2 proteins,” explained Kim. “But if we delete them in cells, the cellular growth rate is significantly influenced; some cell lines don’t survive, making it difficult to attribute results to the stress granule functions of G3BP1/2 or their other cellular functions.” These inhibitors will allow researchers to probe stress granules without a complete system-wide disruption.
With this potential in hand, the researchers explored whether stress granules are directly involved in pausing translation under stress or whether stress granules form after translation has halted, a hotly contested subject in the field. “We never understood which comes first,” said Kim. “It’s like the chicken or the egg situation.”
The researchers demonstrated that inhibition of G3BP1 does not influence translation rates in cells. “We inhibit it, we lose stress granules, but we see there’s no rescue of translation,” explained Freibaum. “It puts a little stronger nail in the coffin of the idea that stress granules are intimately linked with inhibiting translation.”
A tool to investigate condensate drugging
There is still much to learn about RNP granules, especially their links to disease. The nature of how they manifest means that directly observing their connection with neurodegenerative disorders is currently impossible.
“In terms of understanding granule formation in living organisms, we are at a very early stage,” said Kim. “There are links between granule formation and pathology, but we don’t really understand if this pathology is coming from those granules.”
Targeted inhibitors are key to filling these knowledge gaps, and as Taylor emphasizes, the entire condensate landscape benefits from the lessons learned here. “Beyond the generation of tools for studying stress granules,” he said, “this work provides proof-of-concept that understanding the constituents of condensates and their relative contribution to condensation provides a blueprint for manipulating these structures for therapeutic purposes.”
The addition of G3BP inhibitors is a game-changer. This discovery will allow for more rigorous investigations to address the connection between RNP granule formation and disease pathogenesis and whether targeting condensate formation is a viable route to therapy.






