St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
On the Road to Discovery: How Understanding Mitochondrial Trafficking Drives Change
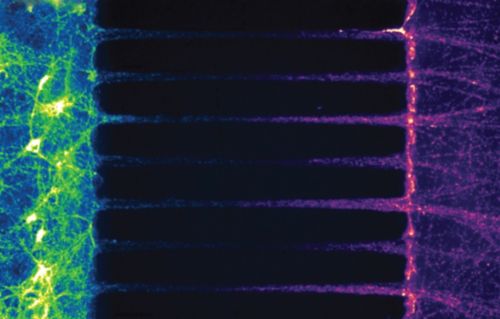
Staining mitochondria (green for soma, and red for axonal) in rat hippocampal neurons grown in a microfluidic chamber shows how mitochondrial trafficking is asymmetrical in axons – which could be linked to quality control in this organelle.
Science is complicated; there is no way around it. Every time a scientist answers one question, a dozen more pop up. It’s the classic “dog chasing its tail” scenario, where there is no beginning and no end. But that’s the beauty of science — there is a never-ending treasure trove of exciting phenomena to explore, and, in the process, we learn about ourselves and the world around us.
However, science is not just about asking a “good” question; it’s about asking the “right” question and knowing the difference between the two. It truly is an art form. Jason Vevea, PhD, Department of Developmental Neurobiology, recently asked one of those “right” questions and reminded us that mitochondria are more than the “powerhouse of the cell.”
More than a powerhouse
If you search for publications about mitochondria, 10,876 PubMed entries show up in 2022 alone. They are extensively researched organelles due to their heavy hand in so many cellular processes. However, if you search for “mitochondria” and “neuron,” only 1,140 entries return. This result is baffling, considering that mitochondrial dysfunction is a feature of almost every neurodegenerative disease studied.
In healthy neurons, mitochondria travel back and forth from the soma (cell body), across the axon and into the synaptic terminal (end of the neuron), delivering ATP (energy) and promoting cell signaling. In disease, damaged mitochondria fail to return to the soma, where lysosomes degrade them. Think of it like abandoned cars on the axon highway; the cars or damaged mitochondria just sit there and eventually cause the cell to get sick.
So, how do healthy neurons keep damaged mitochondria from stalling out on the axon highway? This is a question Vevea was ready to answer. As a graduate student in the laboratory of Liza Pon, PhD, at Columbia University, Vevea developed a keen interest in organelle quality control, or how a cellular structure like a mitochondrion maintains optimal performance. The Pon laboratory uses the model organism Saccharomyces cerevisiae (yeast) to study mitochondrial trafficking and inheritance, and it was during his time as a graduate student that Vevea helped identify mitochondria as an aging determinant. He also helped show that this depended on successful trafficking and accumulation of mitochondria in the dividing cell.
Vevea always wondered if something similar influenced the health of mitochondria in the axon of neurons. Since axons cannot make mitochondria, they must travel from the soma. So, Vevea wondered whether this trafficking was involved in quality control.
“I wanted to know if this trafficking-based quality control mechanism is present in the axons of mammals. Is this cellular process conserved from single-cell yeast to mammalian neurons?” Vevea said.
The answer was yes.
Vevea put neurons in a microfluidic device and stained axonal mitochondria with a red dye and the mitochondria in the soma with a green dye. Over time, he saw mitochondria going to and from the axon, and he noticed that normal mitochondria showed direction-dependent differences in size and health — those heading toward the synaptic terminal were longer and healthier than those heading back to the soma. Vevea hypothesizes that these directionally dependent differences are tied to quality control measures inside the axon.
Touch and go
Vevea also observed biochemical mitochondrial complementation in the neuronal axon during these time-lapse trafficking experiments. Complementation happens when two mitochondria physically touch — a process called fusion — and mix their contents. It is a way that a healthy mitochondrion can theoretically aid another mitochondrion showing signs of damage.
Specifically, Vevea confirmed complementation by looking at the neuronal redox state, which measures a cell’s electron balance. The observed redox complementation was the first of its kind, thereby advancing the field and highlighting how multifaceted mitochondria truly are.
Trafficking and disease
These findings, published in the Journal of Neuroscience, also detailed the importance of mitofusin 2 (MFN2), a protein that helps mitochondria fuse, in modulating calcium. Insufficient MFN2 in neurons derailed mitochondrial trafficking. These mitochondria also took up more calcium — an ion important in signal transduction in the brain.
Knowing that mutations in MFN2 are linked to diseases, Vevea thinks that when the traffic pattern is disrupted in cells because of a problem with MFN2, they may get stuck in the wrong place and, for some reason, take up too much calcium. This excessive calcium may eventually cause abnormal mitochondrial calcium uptake and perpetuate mitochondrial dysfunction. This is one of many interesting questions Vevea is following up on as he starts his new laboratory at St. Jude.
The age-old question: how do we age?
The body is an interesting machine; one day, we start the engine, and if we’re lucky, it doesn’t turn off for decades. But what happens to our engine over time? What changes, specifically in the brain, occur as we age but also in disease? This line of questioning is particularly fascinating to Vevea.
The Vevea lab focuses on understanding the quality control mechanisms used in neurons to keep them firing for decades. He specifically focuses on the synaptic vesicle (SV) cycle — the sequence of membrane-trafficking events of vesicles in the presynaptic terminal, which prepare and execute neurotransmitter release. This cycle is crucial for how neurons transmit signals in the brain, and its functionality relies, in part, on healthy mitochondria. However, the cycle breaks down with age and in neurodegenerative conditions, and scientists still don’t know all the proteins involved in it and what changes may happen to these proteins over time and in disease.
“If we can isolate these proteins, protein complexes, and organelles based on time, we could better understand the molecular changes that happen as we age or in neurodegenerative diseases. A comparative biology approach based on time will lead to new areas of research and possibly new therapies,” says Vevea.
Simply put, understanding why mitochondria break down on the axon highway and knowing who is commuting on this axon highway will help narrow down potential targets for aging-related issues and neurodegenerative diseases.
A new approach in a new field
Studying cellular quality control in neurons has been historically difficult. However, with new advancements and methodologies, Vevea believes now is the time to ask detailed questions about how a neuron maintains itself and its organelles throughout its lifespan.
Vevea is in the driver’s seat, ready to explore this uncharted territory using advanced microscopy and novel techniques he has developed. His unique technical approaches, along with his creative mindset, will push the boundaries in terms of what we know about developmental neurobiology and provide valuable resources for other scientists.
Vevea states, “Now that we have demonstrated heterogeneity in mitochondria and in neurons, we can build on this. I want to better understand what physically makes mitochondria different over time. We will use a discovery type of experiment where we leverage some of the new pulse-chase affinity tag technology we have been working on. I’m very excited about what we may discover and how these discoveries may play a role in understanding aging and disease.”






