St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
Research points to a way to reduce cachexia, a cancer-induced muscle wasting disorder

Fabio Demontis, PhD, led research into the muscle wasting disorder cachexia in a pediatric laboratory model.
A bald head from the effects of chemotherapy is often the most visible sign that a person has cancer. Another tell-tale sign is the dramatic loss of body weight, muscle mass and body fat that leaves a person thin and frail. This “wasting” disorder, called cancer-induced cachexia, occurs in some of the most malignant cancers and is estimated to be responsible for 20–30% of cancer deaths.
“Cachexia is a problem that many patients with cancer experience, which causes not only a decrease in muscle mass but a functional decline in many organ systems,” said Fabio Demontis, PhD, of the St. Jude Department of Developmental Neurobiology. “Laboratory research suggests that if you can treat cachexia you can extend life expectancy and improve patients’ response to cancer therapy, even if you don’t stop cancer growth per se.”
Cachexia is caused by inflammatory signals sent from cancer cells to tissues such as skeletal muscles. The extent of wasting that ensues depends on many factors, including signaling and anabolic factors produced by affected tissues. The Demontis lab studies how skeletal muscle communicates with the body using muscle-secreted signaling factors called myokines.
In recently published research in Nature Communications, the Demontis lab showed that many myokines are necessary to maintain the size of muscle cells (myofibers) and to protect them from wasting induced by cancer cells.
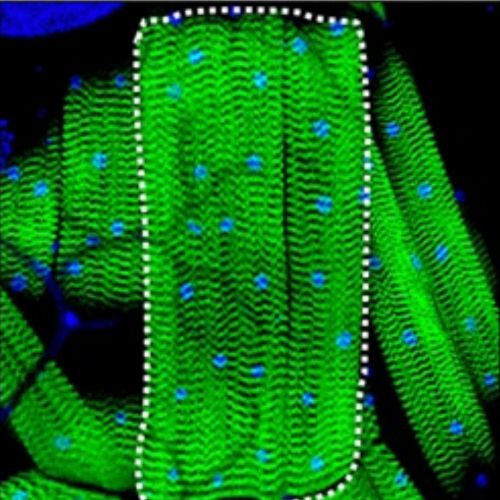
Researchers use fluorescent microscopy to capture images of muscle size induced by myokine overexpression in Drosophila.
To tackle a problem, first you need the right model
Cachexia is a well-recognized problem for adults with cancer. While it can occur in children, it has not been well studied in pediatric oncology. Due in part to a lack of awareness about pediatric cachexia, the lack of research is also due to an insufficient research model of the disorder.
“There has been a lot of research on cachexia in adult cancers, but in terms of pediatric cachexia there is very little,” Demontis said. “It’s been observed in the clinic particularly for children with some solid tumors but there has been very little systematic research.”
Demontis and his team use Drosophila melanogaster (fruit flies) as models for screening genes and determining their importance in the regulation of muscle-cell size. For this research, the Demontis lab used fruit flies as well as a model of pediatric cachexia based on patient-derived xenografts.
The researchers used cancer samples from a pediatric melanoma patient treated at St. Jude. In the mouse models, the pediatric melanoma cells induced around 25% muscle wasting, making it a robust pediatric cachexia model.
The model is freely accessible to others through the St. Jude Childhood Solid Tumor Network (CSTN). CSTN offers the world’s largest and most comprehensive collection of scientific resources for researchers studying pediatric solid tumors and related biology.

First author Flavia Graca, PhD, and study authors Liam Hunt, PhD, and Anna Stephan studied how muscle signaling is involved in cachexia.
A clue in the signals that tell muscles to grow
With a model of pediatric cachexia, the researchers were able to study the signals involved in muscle wasting in the context of this disorder. Myokines can also act as signal throughout the body or more locally, effecting the size of muscle cells.
The researchers conducted an experiment where they “knock down” or remove genes systematically by a process called RNA interference (RNAi) to find genes that are needed to achieve optimal muscle cell (myofiber) size. The scientists found that the myokine Fibcd1 helps preserve myofiber size. Fibcd1 is found in fruit flies, mice and humans. The researchers used a lab-produced (recombinant) Fibcd1 protein made by the St. Jude protein production facility to reduce myofiber atrophy in their pediatric cachexia model.
In particular, they looked at the effect of recombinant Fibcd1 on the diaphragm, a key muscle necessary for respiration and an overall determinant of fitness. The researchers found that in the cachexia models, Fibcd1 could reduce wasting of the diaphragm muscle cells, and also improve exercise capacity. By addressing cachexia, health care providers might help patients better tolerate their cancer therapy and improve survival and quality of life. These findings could also be useful for understanding or treating other muscular or inflammatory disorders that affect the diaphragm such as infections and aging.
“Injecting recombinant Fibcd1 was able to partially rescue the decline in muscle size induced by cancer cells,” Demontis said. “It’s not a complete solution to cachexia, but it’s showing that this approach of exploiting myokines could be helpful for targeting key tissues such as the diaphragm that are important for survival.”
Future opportunities for research
Fibcd1 seems to signal via a receptor present on muscle cells but not on cancer cells, so adding Fibcd1 as a treatment for muscle wasting is unlikely to fuel cancer growth. While these findings have promise, there is currently no clinical therapy that uses Fibcd1 to treat cachexia. More research is needed to demonstrate the efficacy and safety of such an approach.
The model created by Demontis and his team will also be instrumental in continuing studies to better understand the mechanistic basis of pediatric cachexia.
“This model could also be used to test other treatments for cachexia, such as repurposing drugs that have already been developed for other conditions,” Demontis said.
The study’s first author is Flavia Graca, PhD, a postdoctoral researcher in the Demontis lab. The study was primarily supported by a grant from the Hartwell Foundation and by developmental funds from the St. Jude Comprehensive Cancer Center and ALSAC, the fundraising and awareness organization of St. Jude.






