St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
CDK6: A well-known kinase commits a new ‘crime’ connecting outer radial glia to microcephaly
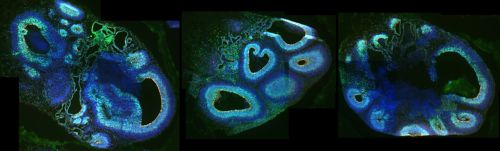
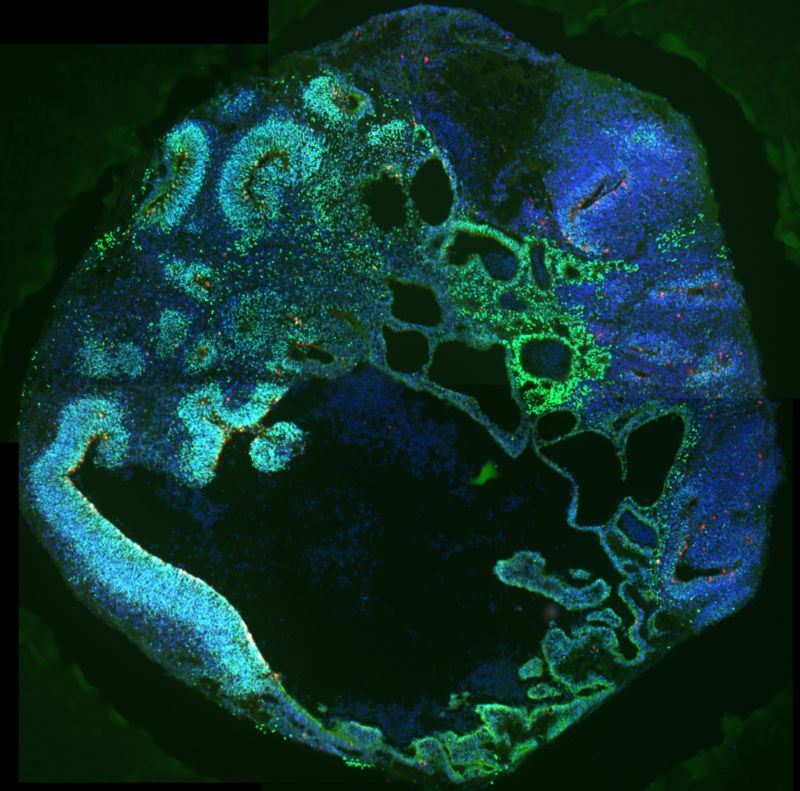
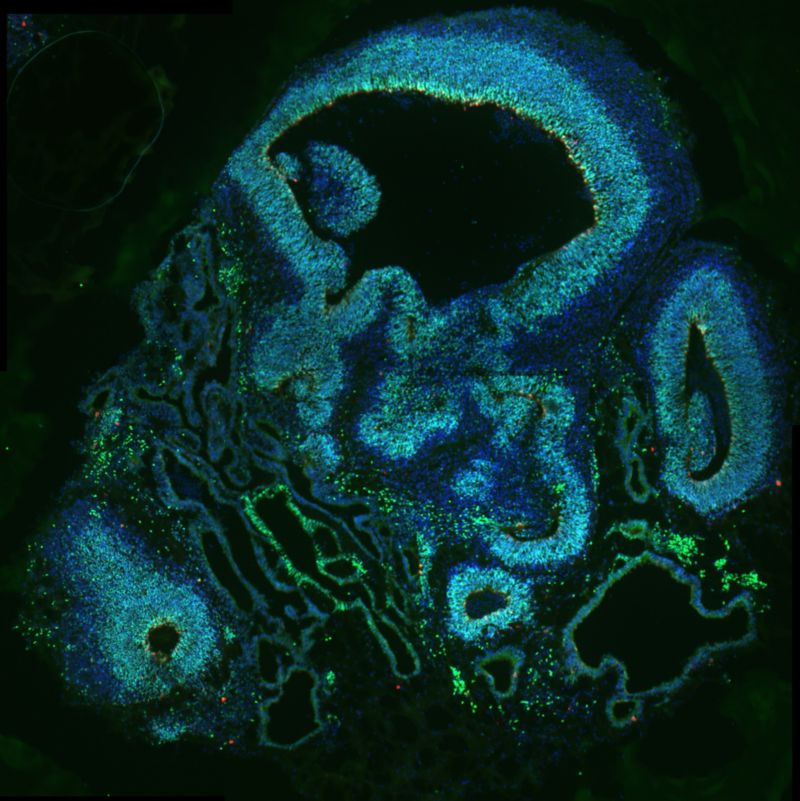
Cerebral organoids, models used for research, made of neural progenitor cells with mutated CDK6 and stained to reflect the expression of different factors including PAX6 (green), phospho-vimentin (red), and DAPI (blue). The models were used in the Han laboratory’s work on CDK6, outer radial glia and microcephaly.
For all that science and technology have revealed about the human brain and how it works, there are still many processes in the brain that remain mysterious. At St. Jude, developmental neurobiologists are helping to unravel these mysteries to better understand how the brain forms and functions.
Microcephaly is a neurodevelopmental condition in which a baby’s head is much smaller than expected because the brain did not develop properly or stopped developing during pregnancy. The condition can lead to intellectual disabilities, speech delays, seizures and abnormal muscle function. There is no cure for microcephaly, and cases are treated with symptom management and support services.
Microcephaly is rare, with about 25,000 cases in the United States each year. The causes include infections, malnutrition and exposure to toxins. For example, in 2016 a surge in Zika virus infections led to an increase in cases of microcephaly around the world.

Young-Goo Han, PhD
Young-Goo Han, PhD, St. Jude Department of Developmental Neurobiology, is studying what goes awry in the developing brain that can lead to microcephaly.
“In babies with microcephaly, the cells that make the brain were not produced in the correct numbers during development,” Han explains. “The brain is smaller because there are fewer neurons and glial cells, which should have been made from progenitor cells during development.”
The primary progenitor for most cells in the brain are called ventricular radial glia. However, outer radial glia cells are another major type of progenitor that produces neurons. Like ventricular radial glia, outer radial glia produce neurons directly or through intermediate progenitor cells.
Han and his team studied how the protein CDK6 functions in outer radial glia, demonstrating that these cells play an important role in microcephaly. CDK6 is a key signaling protein that is well known in biology for its critical role in the cell cycle and contributions to diseases such as cancer. Han has demonstrated that CDK6 commits a new ‘crime’ connecting outer radial glia to microcephaly.
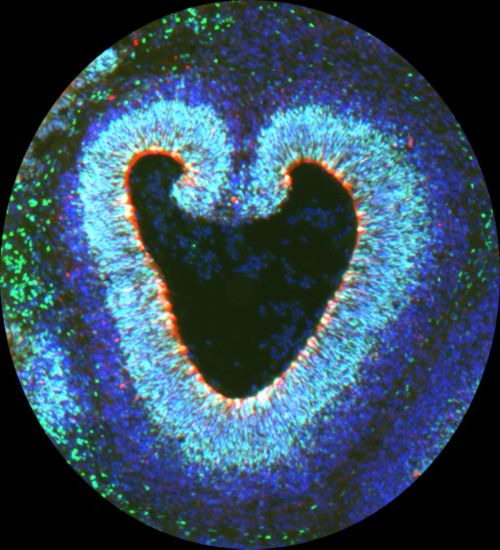
CDK6 and outer radial glia affect brain folding
The brain often evokes images of cauliflower because both have textured surfaces and folds. Han and his colleagues recently reported in Proceedings of the National Academy of Science that outer radial glia are responsible for those characteristic folds in the brain due to the activity of CDK6.
Outer radial glial cell populations are greatly expanded in species that have large, folded brains such as humans or monkeys, but these cells are rare in species that have small and smooth brains like mice or rats.
“We suspected that outer radial glia had an important role in making the brain larger and folded, but this hadn’t been fully explored,” said Kris Olesen, PhD, Developmental Neurobiology. “There are many genes whose dysfunction can cause microcephaly. But outer radial glia are an underappreciated piece of the puzzle.”
It’s difficult to study outer radial glia in the lab because the populations of these cells are so small in many commonly used animal models. While it was known that mutations in CDK6 can cause microcephaly in humans, it does not in mouse models. This led to the hypothesis that outer radial glia, which are very infrequent in mice, may be the missing piece connecting CDK6 and microcephaly.
The right model leads to discovery
In 2016, Han and his team published their work to create the first genetically modified animal model of outer radial glia for laboratory research. Using that model, they were able to determine that outer radial glial cells are important for the development of the larger, folded brain. They were also able to understand how CDK6 affects the outer radial glia and their role as progenitor cells.
CDK6 is a kinase (enzyme) that interacts with other target proteins and has been studied for its role in the cell cycle. Han and his team were surprised by the new role they discovered for CDK6. They thought it might regulate the proliferation of neural progenitor cells, but instead found that CDK6 regulates self-renewal of these progenitor cells. Self-renewal is an aspect of development whereby progenitor cells don’t differentiate (assume an adult identity and function) and instead refresh themselves as progenitors so that they can keep giving rise to other cells.
“We found that CDK6 is necessary for these outer radial glial cells to self-renew, independent of the other well-known functions of this important kinase,” said Fengming Liu, PhD, Developmental Neurobiology.
“I think in the future, we will be able to use this model to get a much clearer picture of the functions of outer radial glia, and how they might contribute to developmental disorders, which we haven’t been able to study with conventional models,” Han said.
-
Slide activated
-
Slide activated