St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
Changing the trajectory of flu for the sickest patients
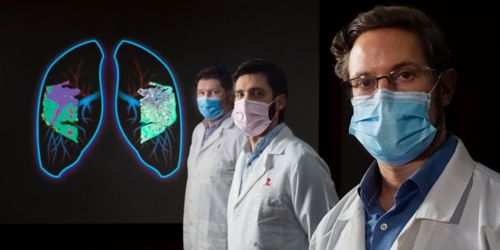
Research reveals that supportive tissue in the lungs helps coordinate the immune response to flu by regulating access to the tissue and inflammation in the lungs. Pictured are co-author Jeremy Chase Crawford, PhD, left; first author David Boyd, PhD, center; and corresponding author Paul Thomas, PhD.
Influenza and flu-related pneumonia remain leading causes of death in the U.S. Health officials estimate flu has killed between 12,000 and 61,000 residents annually since 2010.
The deaths often occur when patients’ immune systems have defeated the flu virus and the infection is winding down. But there is no effective therapy for life-threatening lung damage caused by inflammation, which is part of the immune response. Patients must rely on supportive care such as mechanical ventilators. Tamiflu and other drugs can speed flu recovery, but they must be given within days of the first symptoms.
So, how can we tame or reduce inflammation and prevent flu deaths?
I am a postdoctoral fellow in the St. Jude Children’s Research Hospital laboratory of Paul Thomas, PhD, in the Immunology department. For the past decade, that question has been a lab focus.
I am also first author on research from the Thomas lab that points to a possible answer. The work appeared recently in the journal Nature.
Immunity and flu: Finding a balance
A successful immune response requires a delicate balance. The response must eliminate the pathogen, in this case the flu virus, without causing collateral damage.
Previous research showed that infiltrating immune cells responding to the flu infection damage the lungs. How these immune cells get into the lung and the structural changes that occur with damage were unclear. By focusing on the interface between the immune response and lung function, we identified a mechanism and a promising treatment approach.
Mechanism driving the flu immune response
We demonstrated that during respiratory infections such as flu, fibroblast cells serve as the gatekeepers of the inflammatory immune response in the lungs. Fibroblasts also function as the central hub regulating the outcome of lung function following respiratory infections. That was a surprise. Fibroblasts are best known as cells that build the connective and supportive tissue in organs, including the lungs. The cells have been associated with rheumatoid arthritis and neurological development, but not lung function. This research showed they also help coordinate the immune response by regulating access to the tissue and inflammation in the lungs.
We demonstrated that flu infections can activate a subset of fibroblasts, which we called damage-responsive fibroblasts. These cells pump out molecules called cytokines that drive inflammation. The cells also increase production of the enzyme ADAMTS-4. ADAMTS-4 degrades a key lung protein, versican. The changes promote inflammation and widespread restructuring of lung tissue at the expense of lung function.
When we eliminated ADAMTS-4, mice with flu infections lived longer. A check of ADAMTS-4 concentrations in children and adults hospitalized at three different medical centers across the globe revealed enzyme levels were a strong predictor of flu severity. Those with higher levels of ADAMTS-4 were more likely to need mechanical breathing support and were at greater risk of death.
We used several cutting-edge genomic technologies, including single-cell and spatial transcriptomics to generate ‘big data’ and take a closer look at what was happening in the lungs. Co-author Jeremy Chase Crawford, PhD, led the data analytics and technology development for this work. The approach showed that fibroblast activation was associated with severe lung damage and that the activation and damage were different in different lung regions. The damage was also associated with the presence of ADAMTS-4.
A possible strategy to block virus-related lung damage?
An ADAMTS-4 inhibitor has attracted attention as a possible rheumatoid arthritis treatment. Now we are exploring small-molecule inhibitors to limit excessive inflammation and lung damage in patients with respiratory infections. The approach could fill a treatment gap for patients with flu and other respiratory infections, offering effective treatment days after the Tamiflu window has closed.
The Thomas lab is exploring the possibility that the same mechanism for ADAMTS-4 is at work in asthma and other respiratory infections, including COVID-19. The need for new treatments that specifically reduce lung damage and improve outcomes has been discussed in the influenza field for decades. The current COVID pandemic has highlighted this need for new host therapeutics and brought this area of research to an even wider audience.






