St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
The SILver Lining: The yin-yang balance of proteins in muscle disease

Linda Hendershot’s lab recently elucidate the molecular mechanisms causing muscle disease in Marinesco-Sjögren Syndrome using a preclinical model.
Nearly two decades ago, our lab discovered the human version of SIL1, a protein that plays a key role in the biological origami of protein folding. A few years later, as part of a larger collaboration, our lab and another group identified mutations in SIL1 as the root cause of Marinesco-Sjögren syndrome.
What is protein folding?
As the name suggests, protein folding is the process by which a newly synthesized protein changes from a string of amino acids into its mature 3-D, functional shape. This process is governed by catalyst proteins called molecular chaperones, which ensure that the new protein folds correctly. BiP is a crucial chaperone that resides within the cell’s endoplasmic reticulum (ER) compartment. BiP’s function is regulated by a set of its own helper proteins, such as SIL1.
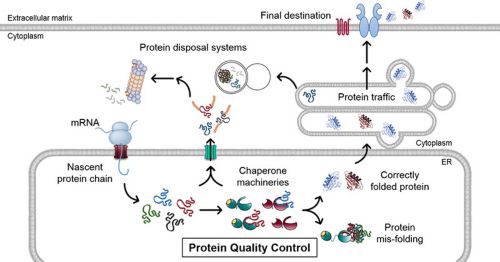
Nearly a third of a cell’s proteins are synthesized within the endoplasmic reticulum (ER). Here, molecular chaperones and their helper proteins ensure that new proteins fold correctly, so they can continue their journey to their final destination. However, protein folding is not a fool-proof process and when the new proteins misfold, these same chaperone machineries recognize the misfolded proteins and target them for degradation by the cellular protein disposal systems. This constitutes the protein quality control process within the ER, and BiP is a key chaperone that lies at its epicenter.
What is Marinesco-Sjögren syndrome?
Marinesco-Sjögren syndrome (MSS) is a rare but debilitating childhood disease that causes juvenile cataracts and a severe and progressive loss of muscle strength and coordination. In many cases, these conditions are also accompanied by intellectual disability, growth deformities and other symptoms. People with MSS have a normal life expectancy. In these patients, genetic defects in the SIL1 gene generally result in a negligible amount of functional protein. That situation likely compromises BiP’s ability to function normally.
What led us to study the role of SIL1 at the crossroads of MSS?
During the past three decades, St. Jude molecular cell biologist Linda Hendershot, PhD, has made strides in understanding how different proteins fold within the ER and how chaperones function in this process, in health and in disease. MSS and cancer are diseases that have puzzled scientists in our lab. The link between mutations in SIL1 and MSS has been well established.
When I joined the lab as a doctoral student in 2012, my mission was to answer two questions:
- How do MSS-associated genetic mutations lead to the loss of SIL1’s function?
- How does the loss of that function lead to the disease’s symptoms?
Each discovery over these years has uncovered new questions and has led us closer to a better understanding of SIL1 and MSS. In our most recent work, collaborating with other groups at St. Jude, we learned it was all about the yin-yang balance of proteins.
Loss of SIL1 wreaks havoc
Cells have numerous protein-folding industries distributed throughout their compartments. SIL1, BiP and other components of the cell’s protein-folding machineries within the ER play an essential role in many aspects of a protein’s journey from biogenesis to degradation. The ER is also connected to other cellular compartments and represents a node within the cellular network. Thus, the loss of SIL1, as in the case of MSS, dramatically disrupts protein homeostasis within the ER, which causes a cascading ripple effect that affects other cellular compartments.
(Hu)Man, mouse and muscle
We use a preclinical model to help us better understand the underlying disease mechanisms of MSS. Recently, we focused on the progressive loss of muscle mass and accompanying muscle weakness, which are hallmarks of MSS. We reasoned that understanding the molecular changes leading to muscle dysfunction would provide insights into many other pathological aspects of MSS. Unlike nerve cells—which are vulnerable to stressful conditions, as exemplified in many neurodegenerative diseases—muscles are more robust and allow us to photograph the changes leading up to the onset of muscle weakness and the subsequent disease progression.
Going to war
When protein homeostasis is disrupted within the ER, cellular SWAT teams (chaperones and protein disposal systems) repeatedly attempt to salvage the situation and prevent further toxicity. When these actions do not resolve the defects, cellular disposal systems are heavily taxed. Those stresses begin to impinge on protein homeostasis beyond the ER. Now, the cell begins losing the war.
Little leaks sink the ship
Nearly a third of the proteins encoded by the mammalian genome move through the ER. Many of these proteins rely on the BiP machinery for biogenesis. When BiP function is compromised, the maturation of ER-trafficking proteins can be affected. We found that the loss of SIL1 affected the maturation of two such proteins, insulin receptor and insulin-like growth factor 1 (IGF-1) receptor. These cell-surface receptors ‘sense’ the circulating levels of their intended targets, insulin and IGF-1, and relay that information as signals. Within muscles, these receptor-driven signals play a crucial role in controlling the uptake and metabolism of sugars and in controlling protein homeostasis. This leads to secondary and tertiary consequences, reminiscent of the cascading ripple effect mentioned earlier.
The SILver lining
What makes this research exciting to our lab is that we are able to understand the molecular events underlying muscle weakness in the preclinical model lacking SIL1. We believe a similar ripple effect could also trigger the collapse of protein homeostasis in individuals with MSS. After identifying the molecular milestones of the progressive loss of muscle mass and strength, we now pose a new question: “How can we treat these defects?”
Citation: Ichhaporia, V. P., et al. (2018). "SIL1, the endoplasmic-reticulum-localized BiP co-chaperone, plays a crucial role in maintaining skeletal muscle proteostasis and physiology." Disease Models & Mechanisms 11(5).






