St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
Yunlong Zhao Lab
Decoding the spatial orchestration of T-cell co-receptor signaling to reshape anti-tumor responses
About the Zhao lab
Cancer immunotherapy has revolutionized treatment by employing various strategies to boost the immune system, including harnessing T-cell co-receptor signaling. However, this innovative therapy benefits only a subset of patients. A key to broadening its impact lies in unraveling the complex web of signals that control co-receptors. In our laboratory, we explore the spatial regulation of T-cell co-receptor signaling, focusing on both cis (within the same cell) and trans (between cells) interactions. By decoding this spatial orchestration, we aim to enhance immunotherapy, revealing new ways to trigger stronger anti-tumor responses and expand treatment benefits to more patients.

Activated T cell
Our research summary
T cells are essential players in the immune system, continuously surveying tissues for signs of pathogens or tumor cells. Properly regulated T-cell responses are key to eradicating harmful cells while avoiding excessive immune activation. Therefore, understanding the intricacies of T-cell signaling and its regulatory mechanisms is critical for designing effective therapies to treat cancers and catastrophic diseases.
Immune cells, like T cells, communicate through dedicated co-receptor signaling pathways. Historically speaking, these signaling pathways were thought to primarily take place through trans-interactions – communication between cells via ligands and receptors on different cell membranes. However, our studies reveal the significance of cis-interactions – cell-intrinsic communication between receptors and ligands on the same cell membrane. These two dimensions of signaling—cis and trans—are not isolated events but tightly interwoven, creating a spatially regulated network that controls T-cell activity.
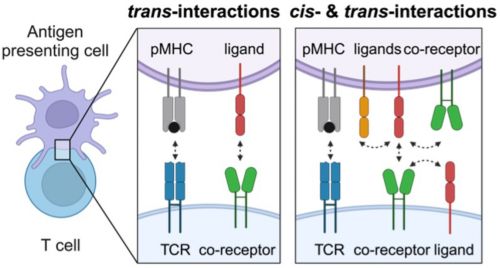
Illustration of cis- and trans- interactions in T-cell signaling
We aim to understand the spatial orchestration of these interactions. How do T cells integrate signals across the membrane landscape? How does the positioning of receptors and ligands in two-dimensional space dictate T cell function? By decoding this spatial regulation, our goal is to translate these findings into therapeutic strategies, ultimately improving patient outcomes.
Cis-interactions and immune checkpoints

The T-cell response is tightly regulated by immune checkpoints, such as CTLA-4 and PD-1. CTLA-4 is constitutively expressed on regulatory T cells and downregulates immune responses when bound to CD80 and CD86 ligands, while PD-1 interaction with PD-L1 suppresses the killing of tumor cells by T cells.
Our research discovered that PD-L1 interacts with CD80 in cis and, further, that cis-PD-L1:CD80 interactions can rewire trans-signaling pathways by inhibiting the binding of PD-L1 with PD-1 and CD80 with CTLA-4, while maintaining the ability of CD80 to interact with another of its cognate stimulatory receptors, CD28. Therefore, cis-PD-L1:CD80 interactions modulate the balance between inhibition (PD-1 and CTLA-4) and stimulation (CD28). This finding opens new possibilities for therapies that shift immune responses from suppression to activation, leading to more effective cancer treatments.
Membrane invagination-mediated cis-CD28 stimulation

CD28 is a co-stimulatory T-cell receptor that responds to binding CD80 and CD86 (B7 ligands). However, even though these ligands are not abundantly expressed in peripheral tissues, CD28 co-stimulation of T cells in peripheral tissues still occurs. Our laboratory provided evidence that through cis-interactions, B7 ligands on CD8+ T cells engage CD28 at membrane invaginations of the immunological synapse, resulting in a self-sustaining co-stimulatory mechanism. This finding points to cis signaling as an important modulator of T-cell functionality.
Selected Publications
About Yunlong Zhao
Dr. Zhao received his PhD in cell signaling in host-pathogen interactions from the Institute of Microbiology, Chinese Academy of Sciences in Beijing. He then completed his postdoctoral fellowship at the University of California, San Diego, where he focused on T-cell signaling. Dr. Zhao joined St. Jude as an Assistant Member in the Department of Immunology and is a key contributor to the Center of Excellence in Pediatric Immuno-Oncology. His research focuses on T-cell co-receptor signaling, specifically the influence of spatial regulation on T cell anti-tumor responses.

Contact us
Yunlong Zhao, PhD
Assistant Member, St. Jude Faculty
Department of Immunology
MS 1160, Room I6113
St. Jude Children's Research Hospital

Memphis, TN, 38105-3678 USA GET DIRECTIONS