St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
Mittag Lab
Investigating mechanisms underlying biomolecular phase separation and how they determine functional and pathological processes
About the Mittag Lab
Proteins can associate into discrete complexes, clusters of variable size or even condensates. The latter represents a separate phase with dynamic, fluid-like properties. Such condensates formed via phase separation compartmentalize cells and regulate biological functions. Our work aims to understand the biophysics underlying such phenomena to help unlock pathological mechanisms of cancer and neurological disorders
Our research summary
The Mittag Lab focuses on understanding the molecular interactions underlying the formation of membraneless organelles, also called biomolecular condensates, and how they give rise to function and disease. We have made critical contributions to the conceptual understanding that biomolecular condensates form through phase separation and that multivalent interactions in disordered proteins and domain-motif interactions mediate through this process.
Our current studies specifically focus on the physical properties of biomolecular condensates, sequence-conformation-function relationships of IDPs and disease processes caused by dysregulated phase separation. We employ a combination of biophysical, biochemical and cell biological approaches in our research, as well as specialized tools like NMR spectroscopy, light scattering, fluorescence approaches, microscopy and proteomics.
Phase separation and biomolecular condensates
An increasing number of biomolecular condensates — including the nucleolus, stress granules and nuclear speckles — have been shown to form via a phase separation. Importantly, dysregulated phase separation can result in disease. Mutations can disrupt functionally important phase separation, potentially resulting in cancer. Additionally, stress granule dysregulation can result in aging processes and protein aggregation, giving rise to the hallmarks of neurodegenerative diseases, such as ALS.
Our research will improve our understanding of the formation and function of stress granules, nuclear speckles and condensates involved in transcription, which are the major biomolecular condensates we study. However, the impact of our research reaches further, as phase separation is now recognized to play a role in the DNA damage response, membrane receptor clustering and RNA metabolism.

The role of stress granules in ALS and other neurodegenerative diseases
Stress granules are largely viewed as crucibles for neurodegenerative diseases, and this view is based on histopathological, genetic and cell-biological evidence. Mechanistic biophysical insights show that disease-relevant RNA-binding proteins such as TDP-43, hnRNPA1 and FUS form fibrils earlier in the presence of condensates than in their absence. These data have been interpreted to mean that condensates promote fibril formation and are, therefore, seen as mechanistic support of the crucible hypothesis.
We have recently performed careful thermodynamic and kinetic characterizations of the interplay of condensation and fibril formation and arrived at a fundamentally different interpretation. We showed that fibrils of the hnRNPA1 low-complexity domain are more thermodynamically stable than condensates. While condensate interfaces promote nucleation, fibrils only grow in the dilute phase because condensate interiors suppress the formation of fibrils and, therefore, act as sinks for soluble protein. We propose that stress granules are protective against fibril formation, in contrast to the common view. Ongoing work is further exploring this hypothesis

The role of Myc phase separation in cancer pathogenesis
Initiating and maintaining many cancers requires Myc overexpression. A recent discovery showed that N-myc is diffusely distributed in the nucleus of normal cells, but forms condensates at the elevated levels found in cancer cells, potentially implicating condensates as crucibles for activating oncogenic gene expression programs. While recent work from our lab suggests that phase separation of transcription factors is not required for normal gene activation, transcription factor condensates may mediate additional (gain-of-function) activities through their emergent properties that diffuse transcription factors do not possess. Given that Myc is widely perceived as undruggable and that condensate-spanning protein interaction networks represent attractive new therapeutic targets, understanding Myc condensates’ precise role in cells undergoing oncogenic transformation is paramount.

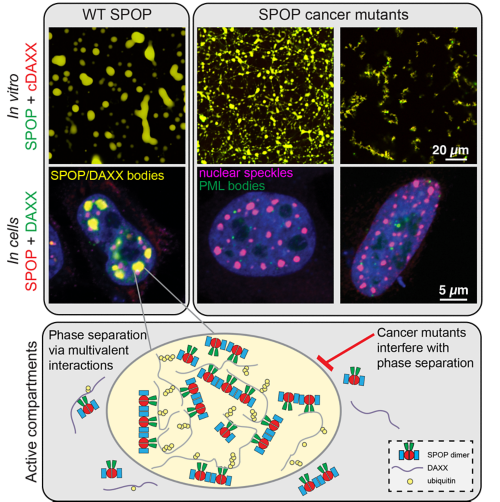
Speckle-type POZ protein localization in biomolecular condensates
The speckle-type POZ protein (SPOP) is a tumor suppressor that is frequently mutated in cancers. It functions as a ubiquitin ligase subunit and is important for maintaining appropriate protein levels of many proto-oncogenes. Prostate cancer-associated SPOP mutations disrupt substrate binding and ubiquitination, which leads to increased levels of oncogenic substrates. By contrast, endometrial cancer-associated SPOP mutations activate substrate turnover abnormally. Our recent structures of SPOP filaments reveal that activating mutations change the quaternary structure of SPOP assemblies, explaining the activating effects and pointing to new mechanisms underlying oncogenesis.
Selected Publications
Contact us
Tanja Mittag, PhD
Structural Biology
MS311, Room M7432
St. Jude Children's Research Hospital
Follow Us

Memphis, TN, 38105-3678 USA GET DIRECTIONS