St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
Richard Lee Lab
Discovering novel therapeutics to treat catastrophic pediatric diseases
About the Lee Lab
Drug-resistant infections are a major problem in patient populations with compromised or suppressed immune systems. Our lab is interested in tackling this problem by applying chemical biology and medicinal chemistry approaches. We encompass multiple skill areas and work closely in interdisciplinary teams within and outside of the lab. Our ultimate goal is to advance new treatments for catastrophic pediatric disease.
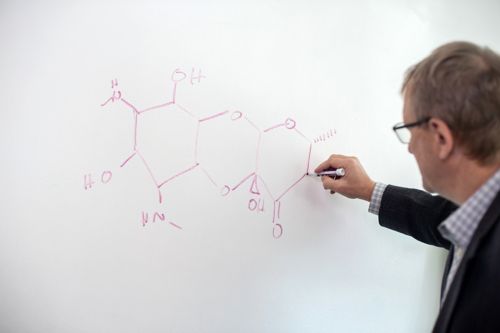
In the news
Innovation and collaboration in drug development helps combat antibiotic resistance
Explore how St. Jude researchers are tackling the silent pandemic of antibiotic resistance and pioneering the development of new drugs to address the growing threat.
Our research summary
Antimicrobial agents
In the antibacterial therapeutics area, the low-hanging fruits for drug discovery are long gone. The major classes of antibiotics, discovered over the past eighty years, are of fundamental importance to the practice of modern medicine and are in danger of being overwhelmed by waves of antimicrobial resistance. Using a historical perspective to understand and tackle this complex problem, we have developed a program that integrates advanced medicinal chemical, biochemical and pharmacological approaches to discover and develop novel antimicrobial agents.

Antibiotic chemistry – Protein synthesis Inhibitors
Ribosome crystallography and Cryo-EM work have enabled the structure-guided design of a new generation of antibiotics. We are currently investigating two series of spectinomycin-derived protein synthesis inhibitors: the spectinamides with proven efficacy against chronic tuberculosis infections, which are uniquely resistant to native efflux in M. tuberculosis; and the aminomethyl spectinomycins, which have a broader spectrum of activity, with proven efficacy against common upper respiratory tract pathogens, atypical bacteria, and non-tuberculous mycobacteria.

Antibiotic chemistry – Targeting drug-tolerant bacteria through ClpP activation
Most antibiotics rapidly kill growing bacteria but are much less effective against non-replicating persistent populations such as those found in biofilms on indwelling devices. Recent studies have indicated that ATP independent activation of ClpP using acyldepsipeptides can kill persistent bacteria. However, Acyldepsipeptides are rapidly degraded in vivo. Using structure-guided drug design, we have discovered and are further developing a new class of ureadepsipeptides that maintain anti-bacterial potency and mechanism but have superior pharmacokinetic properties.
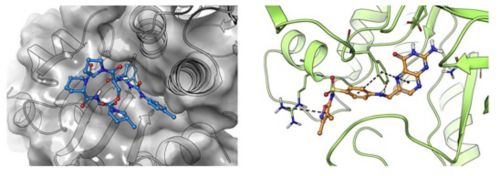
Left: The structure of ClpP from Staphylococcus aureus in complex with ureadepsipeptide (PDB ID 6PKA)
Right: The structure of DHPS from Staphylococcus aureus in complex with antifolate 1530 (PDB ID 6CLV)
Structure-guided design of next-generation antifolates
Antifolates are an important class of antimicrobial drugs whose use is increasingly compromised by drug resistance. To face this challenge, we are developing novel antifolates that inhibit dihydropteroate synthase, the target of sulfonamides, in new ways using structure guided design. To complement this approach, we are applying a nutrient-limited screening approach to find new antimetabolite agents. This work has led to the discovery of a new class of antibiotics that act through Cysteine Synthase A in Gram-negative bacteria.
Discovery of novel enzyme inhibitors
We are interested in the design and discovery of first-in-class inhibitor classes to validate novel therapeutic targets. These targets are often challenging, and we have developed a robust platform to discover novel ligands aided by the outstanding resources found in CBT. Our blended screening approach uses a combination of high throughput and biophysical screening assays (Thermal shift, SPR, ITC, MST, and Crystallography), coupled with rigorous chemoinformatic triage steps and a minimalist approach to subsequent hit-to-lead medicinal chemistry optimization.

Modulators of CoA pathway
The most advanced program in this area is the Pantazine modulators of Co-enzyme A. Co-enzyme A biosynthesis is of fundamental importance to all cells, levels of which are closely controlled by the action of pantothenate kinase. Loss of the PanK2 isoform is associated with a rare neurodegenerative disease PKAN. We have co-discovered and developed the Pantazine class of allosteric activators of the PanK1 and PanK3 isoforms to overcome the loss of PanK2. Our brain penetrant Pantazine leads are a product of our state-of-the-art ligand discovery and advancement platform. This program has produced a lead compound that has received recent approval by the FDA to start clinical trials.
Selected Publications
Contact us
Richard E. Lee, PhD
Chemical Biology and Therapeutics
MS 1000
St. Jude Children's Research Hospital
Follow Us

Memphis, TN, 38105-3678 USA GET DIRECTIONS

